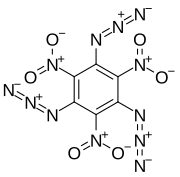
1,3,5-Triazido-2,4,6-trinitrobenzene
| |
| |
| Names | |
|---|---|
| IUPAC name
1,3,5-Triazido-2,4,6-trinitrobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6N12O6 | |
| Molar mass | 336.144 g·mol−1 |
| Appearance | yellow crystals[1] |
| Structure[1] | |
| monoclinic | |
| P21/c, No. 14 | |
| Explosive data | |
| Detonation velocity | 7350 m/s |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its velocity of detonation is 7,350 meters per second, comparable to TATB (triaminotrinitrobenzene).
Preparation
The compound was first synthesized in 1924 by Oldřich Turek.[2] It can be prepared by the reaction of 1,3,5--trichloro-2,4,6-trinitrobenzene with sodium azide, which is obtained from the nitration of 1,3,5-trichlorobenzene with nitric acid and sulfuric acid.[2]
Another route uses the nitration of 1,3,5-triazido-2,4-dinitrobenzene.[1]
Properties
Physical properties
1,3,5-Triazido-2,4,6-trinitrobenzene forms yellow crystalline solids that melt at 131 °C.[3] The crystals are monoclinic with space group P21/c. Its formation is highly endothermic, with an enthalpy of formation of 765.8 kJ/mol and an enthalpy of combustion of 3200 kJ/mol.[1]
Chemical Properties
Even at low temperatures, the compound slowly decomposes by giving off nitrogen gas, converting into benzotrifuroxan. This reaction proceeds quantitatively within 14 hours at 100 °C.[2] As a solution in m-xylene, first order kinetics were observed for the decomposition, with a half-life of 340 minutes at 70 °C, 89 minutes at 80 °C, and 900 seconds at 100 °C.[4]
The compound explodes if rapidly heated above 168 °C.[1]
References
- ^ a b c d e Adam, David; Karaghiosoff, Konstantin; Klapötke, Thomas M.; Holl, Gerhard; Kaiser, Manfred (2002). "Triazidotrinitro Benzene: 1,3,5‐(N3)3‐2,4,6‐(NO2)3C6". Propellants Explos. Pyrotech. 27 (1). doi:10.1002/1521-4087%28200203%2927%3A1<7%3A%3AAID-PREP7>3.0.CO%3B2-J.
- ^ a b c Matyáš, Robert; Pachman, Jiří (2013). Primary Explosives. Springer Science & Business Media. pp. 118–121. ISBN 9783642284366.
- ^ Burov, Yu. M.; Nazin, G. M.; Manelis, G. B. (1999). "Retardation of Monomolecular Reactions in the Solid Phase". Russian Chemical Bulletin. 48 (7): 1250–1254.
- ^ Korsunskii, B. L.; Apina, T. A. (1971). "Kinetics of the Thermal Decomposition of 1,3,5-Triazido-2,4,6-trinitrobenzene in Solution". Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science. 20 (9): 1971–1973. doi:10.1007/BF00854439.


