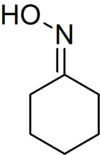Cyclohexanone oxime
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.613 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2811 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H11NO | |||
| Molar mass | 113.16 g/mol | ||
| Appearance | white solid | ||
| Melting point | 88 to 91 °C (190 to 196 °F; 361 to 364 K) | ||
| Boiling point | 204 to 206 °C (399 to 403 °F; 477 to 479 K) | ||
| 16 g/kg (in water) | |||
| -71.52·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Warning | |||
| H228, H302, H319, H373, H412 | |||
| P210, P240, P241, P260, P264, P270, P273, P280, P301+P312, P305+P351+P338, P314, P330, P337+P313, P370+P378, P501 | |||
| Flash point | 110 °C (230 °F; 383 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclohexanone oxime is an organic compound containing the functional group oxime. This colorless solid is an important intermediate in the production of nylon 6, a widely used polymer.
Preparation
Cyclohexanone oxime can be prepared from the condensation reaction between cyclohexanone and hydroxylamine:[1]
- C5H10CO + H2NOH → C5H10C=NOH + H2O
Alternatively, another industrial route involves the reaction of cyclohexane with nitrosyl chloride, which is a free radical reaction. This method is advantageous as cyclohexane is much cheaper than cyclohexanone.
Reactions
The most famous and commercially important reaction of cyclohexanone oxime is its Beckmann rearrangement yielding ε-caprolactam:
This reaction is catalyzed by sulfuric acid,[1] but industrial scale reactions use solid acids.[2]
Typical of oximes, the compound can be reduced by sodium amalgam gives cyclohexylamine.[3] It can also be hydrolyzed with acetic acid to give back cyclohexanone.
References
- ^ a b J. C. Eck and C. S. Marvel "ε-Benzoylaminocaproic Acid" Org. Synth. 1939, volume 19, pp. 20. doi:10.15227/orgsyn.019.0020
- ^ Corma, Avelino; Garcia, Hermenegildo "Organic reactions catalyzed over solid acids" Catalysis Today 1997, volume 38, pp. 257-308. doi:10.1016/S0920-5861(97)81500-1
- ^ W. H. Lycan, S. V. Puntambeker, and C. S. Marvel "n-Heptylamine" Org. Synth. 1931, volume 11, pp. 58.doi:10.15227/orgsyn.011.0058