2,3-sigmatropic rearrangement
2,3-Sigmatropic rearrangements are a type of sigmatropic rearrangements and can be classified into two types. Rearrangements of allylic sulfoxides, amine oxides, selenoxides are neutral. Rearrangements of carbanions of allyl ethers are anionic.[1] The general scheme for this kind of rearrangement is:

Atom Y may be sulfur, selenium, or nitrogen. If Y is nitrogen, the reaction is referred to as the Sommelet–Hauser rearrangement if a quaternary ammonium salt is involved or the aza-Wittig reaction if an alpha-metalated tertiary amine is involved; if Y is oxygen, then it is called a 2,3-Wittig rearrangement (not to be confused with the well-known Wittig reaction, which involves a phosphonium ylide). If Y is sulfur, the product can be treated with a thiophile to generate an allylic alcohol in what is known as the Mislow–Evans rearrangement.

A [2,3]-rearrangement may result in carbon-carbon bond formation. It can also be used as a ring-expansion reaction.[2]

Stereoselectivity
2,3-sigmatropic rearrangements can offer high stereoselectivity. At the newly formed double bond there is a strong preference for formation of the E-alkene or trans isomer product. The stereochemistry of the newly formed C-C bond is harder to predict. It can be inferred from the five-membered ring transition state. Generally, the E-alkene will favor the formation of anti product, while Z-alkene will favor formation of syn product.
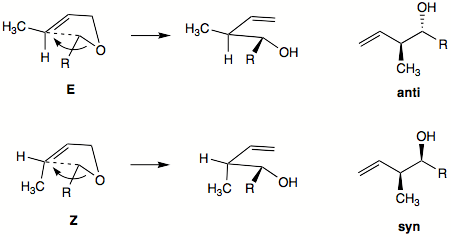
Diastereoselectivity can be high for Z-alkene with alkynyl, alkenyl, or aryl anion-stabilizing group. Diastereoselectivity is usually lower with E-alkenes. Hydrocarbon groups will prefer exo orientation in the envelope-like transition state. Anion-stabilizing group will prefer the endo orientation in transition state.
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Ring expansion by 2,3-sigmatropic shifts of unstabilized sulfonium ylides. Synthesis of eight- to ten-membered thiacycloalk-4-enes V. Cere, C. Paolucci, S. Pollicino, E. Sandri, and A. Fava The Journal of Organic Chemistry 1978 43 (25), 4826-4831 doi:10.1021/jo00419a024
