Saponification
Saponification is a process that involves conversion of fat, oil or lipid into soap and alcohol by the action of heat in the presence of aqueous alkali (e.g. NaOH). Soaps are salts of fatty acids and fatty acids are monocarboxylic acids that have long carbon chains (at least 10) e.g. sodium palmitate.
Saponification of fats
Vegetable oils and animal fats are the traditional materials that are saponified. These greasy materials, triesters called triglycerides, are mixtures derived from diverse fatty acids. Triglycerides can be converted to soap in either a one- or a two-step process. In the traditional one-step process, the triglyceride is treated with a strong base (e.g. lye), which cleaves the ester bond, releasing fatty acid salts (soaps) and glycerol. This process is also the main industrial method for producing glycerol. In some soap-making, the glycerol is left in the soap. If necessary, soaps may be precipitated by salting it out with sodium chloride.

Fat in a corpse converts into adipocere, often called "grave wax". This process is more common where the amount of fatty tissue is high and the agents of decomposition are absent or only minutely present.
Saponification value
The saponification value is the amount of base required to saponify a fat sample.[1] Soap makers formulate their recipes with a small deficit of lye to account for the unknown deviation of saponification value between their oil batch and laboratory averages.
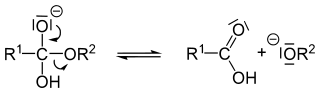
Mechanism of base hydrolysis
The hydroxide anion of the salt reacts with the carbonyl group of the ester. The immediate product is called an orthoester.
Expulsion of the alkoxide generates a carboxylic acid:
The alkoxide ion is a strong base so that the proton is transferred from the carboxylic acid to the alkoxide ion creating an alcohol:
In a classic laboratory procedure, the triglyceride trimyristin is obtained by extracting it from nutmeg with diethyl ether. Saponification to the soap sodium myristate takes place using NaOH in water. Treating the soap with hydrochloric acid gives myristic acid.[2]
Saponification of fatty acids
The reaction of fatty acids with base is the other main method of saponification. In this case, the reaction involves neutralization of the carboxylic acid. The neutralization method is used to produce industrial soaps such as those derived from magnesium, the transition metals, and aluminium. This method is ideal for producing soaps that are derived from a single fatty acid, which leads to soaps with predictable physical properties, as required by many engineering applications.
Applications
Soft versus hard soap
Depending on the nature of the alkali used in their production, soaps have distinct properties. Sodium hydroxide (NaOH) gives "hard soap"; hard soaps can also be used in water containing Mg, Cl, and Ca salts. By contrast, potassium soaps, (derived using KOH) are soft soap. The fatty acid source also affects the soap's melting point. Most early hard soaps were manufactured using animal fats and KOH extracted from wood ash; these were broadly solid. However, the majority of modern soaps are manufactured from polyunsaturated triglycerides such as vegetable oils. As in the triglycerides they are formed from[3] the salts of these acids have weaker inter-molecular forces and thus lower melting points.
Lithium soaps
Lithium derivatives of 12-hydroxystearate and other fatty acids are important constituents of lubricating greases. In lithium-based greases, lithium carboxylates are thickeners. "Complex soaps" are also common, these being combinations of more than one acid salt, such as azelaic or acetic acid.[4]
Fire extinguishers
Fires involving cooking fats and oils (classified as class K (US) or F (Australia/Europe/Asia)) burn hotter than most flammable liquids, rendering a standard class B extinguisher ineffective. Such fires should be extinguished with a wet chemical extinguisher. Extinguishers of this type are designed to extinguish cooking fats and oils through saponification. The extinguishing agent rapidly converts the burning substance to a non-combustible soap. This process is endothermic, meaning that it absorbs thermal energy from its surroundings, which decreases the temperature of the surroundings, further inhibiting the fire.
Oil paints

Saponification can occur in oil paintings over time, causing visible damage and deformation. Oil paints are composed of pigment molecules suspended in an oil binding medium. Heavy metal salts are often used as pigment molecules, such as in lead white, red lead, and zinc white.[5] If those heavy metal salts react with free fatty acids in the oil medium, metal soaps may form in a paint layer that can then migrate outward to the painting's surface.[6]
Saponification in oil paintings was described as early as 1912.[7] It is believed to be widespread, having been observed in many works dating from the fifteenth through the twentieth centuries; works of different geographic origin; and works painted on various supports, such as canvas, paper, wood, and copper. Chemical analysis may reveal saponification occurring in a painting’s deeper layers before any signs are visible on the surface, even in paintings centuries old.[8]
The saponified regions may deform the painting's surface through the formation of visible lumps or protrusions that can scatter light. These soap lumps may be prominent only on certain regions of the painting rather than throughout. In John Singer Sargent's famous Portrait of Madame X, for example, the lumps only appear on the blackest areas, which may be because of the artist’s use of more medium in those areas to compensate for the tendency of black pigments to soak it up.[9] The process can also form chalky white deposits on a painting's surface, a deformation often described as "blooming" or "efflorescence", and may also contribute to the increased transparency of certain paint layers within an oil painting over time.[10]
Saponification does not occur in all oil paintings and many details are unresolved.[11] At present, retouching is the only known restoration method.
See also
References
- ^ "Quality Laboratory Oil Examination Procedures and Practices". American Oil Chemists' Society. Archived from the original on 25 December 2012. Retrieved 17 December 2012.
- ^ G. D. Beal (1926). "Myristic Acid". Organic Syntheses. 6: 66. doi:10.15227/orgsyn.006.0066.
- ^ "Double bonds and hydrogenation". GCSE Bitesize. BBC.
- ^ Bartels, Thorsten; Bock, Wolfgang; Braun, Jürgen; Busch, Christian; Buss, Wolfgang; Dresel, Wilfried; Freiler, Carmen; Harperscheid, Manfred; Heckler, Rolf-Peter; Hörner, Dietrich; Kubicki, Franz; Lingg, Georg; Losch, Achim; Luther, Rolf; Mang, Theo; Noll, Siegfried; Omeis, Jürgen (2003). "Lubricants and Lubrication". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_423. ISBN 3-527-30673-0.
- ^ Groen, Karin (2003). "Pigment". Oxford Art Online. doi:10.1093/gao/9781884446054.article.T067586. Retrieved 2018-01-16.
- ^ Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona (ed.). "The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes". The Metropolitan Museum of Art Bulletin. 67 (1). Metropolitan Museum of Art: 12–19. JSTOR 40588562. See pages: 12–19.
- ^ Fleury, Paul (1912). "MANUFACTURE AND TREATMENTS OF WHITE ZINC". The Preparation and Uses of White Zinc Paints (1st ed.). London: Scott Greenwood & son.
and although Petit declares this theory false, it is none the less on it and on its data that he bases his system of manufacture of hydrated white zinc, of which he is the inventor that is to say, the saponification of the oil, or the formation of metallic salts, dissolved therein
- ^ Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona (ed.). "The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes". The Metropolitan Museum of Art Bulletin. 67 (1). Metropolitan Museum of Art: 12–19. JSTOR 40588562. See page 16.
- ^ Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona (ed.). "The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes". The Metropolitan Museum of Art Bulletin. 67 (1). Metropolitan Museum of Art: 12–19. JSTOR 40588562. See pages 12–13, 15.
- ^ Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona (ed.). "The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes". The Metropolitan Museum of Art Bulletin. 67 (1). Metropolitan Museum of Art: 12–19. JSTOR 40588562. See pages 16, 19.
- ^ Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona (ed.). "The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes". The Metropolitan Museum of Art Bulletin. 67 (1). Metropolitan Museum of Art: 12–19. JSTOR 40588562. See page 19.