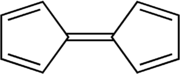
Fulvalene
| |
| |
| Names | |
|---|---|
| Other names
Bicyclopentyliden-2,4,2',4'-tetraene
1,1'-Bi[cylopentadienylidene] Pentafulvalene Bicyclopentadienylidene [5,5']Bicyclopentadienylidene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8 | |
| Molar mass | 128.174 g·mol−1 |
| Density | 1.129 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
History
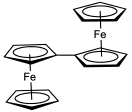
An earlier attempt at synthesis of fulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene.[1] Its synthesis was first reported in 1958 by E. A. Matzner, working under William von Eggers Doering.[2] In this method, cyclopentadienyl anion is coupled with iodine to the dihydrofulvalene. Double deprotonation of dihydrofulvalene with n-butyllithium gives the dilithio derivative, which oxidizes with oxygen. Fulvalene was spectroscopically observed at 77 K from photolysis of diazocyclopentadiene, which induces dimerization of cyclopentadiene-derived carbenes.[3] The compound was isolated in 1986.[4] It was found to be nonaromatic. Above −50 °C it dimerizes by a Diels-Alder reaction.
Derivatives
Perchlorofulvalene (C4Cl4C)2 is quite stable in contrast to fulvalene itself.[5]
See also
- Fulvenes, (CH=CH)2C=CH2 and substituted derivatives
- Tetrathiafulvalene, C2H2S2C=CS2C2H2
References
- ^ T. J. Kealy, P. L. Pauson (1951). "A New Type of Organo-Iron Compound". Nature. 168 (4285): 1039–1040. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0. S2CID 4181383.
- ^ Dissertation Abstracts Int'l 26-06 page 3270 6411876.
- ^ Demore, William B.; Pritchard, H. O.; Davidson, Norman (1959). "Photochemical Experiments in Rigid Media at Low Temperatures. II. The Reactions of Methylene, Cyclopentadienylene and Diphenylmethylene". Journal of the American Chemical Society. 81 (22): 5874–5879. doi:10.1021/ja01531a008.
- ^ Escher, André; Rutsch, Werner; Neuenschwander, Markus (1986). "Synthese von Pentafulvalen durch oxidative Kupplung von Cyclopentadienid mittels Kupfer(II)-chlorid". Helvetica Chimica Acta. 69 (7): 1644 - 1654. doi:10.1002/hlca.19860690719.
- ^ Mark, V. (1966). "Perchlorofulvalene". Organic Syntheses. 46: 93. doi:10.15227/orgsyn.046.0093.
