Cuprate
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−).[1] The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts - oxide materials, anionic coordination complexes, and anionic organocopper compounds.
Oxides
One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2. This species can be viewed as the K+ salt of the polyanion [CuO−
2]n. As such the material is classified as a cuprate. This dark blue diamagnetic solid is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen:[2]
- K2O2 + 2 CuO → 2 KCuO2
Coordination complexes
Copper forms many anionic coordination complexes with negatively charged ligands such as cyanide, hydroxide, halides, as well as alkyls and aryls.
- Copper(I)
Cuprates containing copper(I) tend to be colorless, reflecting their d10 configuration. Structures range from linear 2-coordinate, trigonal planar, and tetrahedral. Examples include dichloro and trichlorocuprates, i.e., linear [CuCl2]- and trigonal planar [CuCl3]2-.[3] Cyanide gives analogous complexes but also the trianionic tetracyanocuprate(I), [Cu(CN)4]3−.[4] Dicyanocuprate(I) exists in both molecular or polymeric motifs, depending on the countercation.[5]
- Copper(II)

The chlorocuprates include trichlorocuprate(II) [CuCl3]−, which is dimeric, square-planar tetrachlorocuprate(II) [CuCl4]2−, and pentachlorocuprate(II) [CuCl5]3−.[1][6] 3-Coordinate chlorocuprate(II) complexes are rare.[7]
Tetrachlorocuprate(II) complexes tend to adopt flattened tetrahedral geometry with orange colors.[8] [9][10][11]
Sodium tetrahydroxycuprate (Na2[Cu(OH)4] is an example of a homoleptic (all ligands being the same) hydroxide complex.[12]
- Cu(OH)2 + 2 NaOH → Na2Cu(OH)4
- Copper(III) and copper(IV)
Hexafluorocuprate(III) [CuF6]3− and hexafluorocuprate(IV) [CuF6]2− are rare examples of copper(III) and copper(IV) complexes. They are strong oxidizing agents.
Organic cuprates
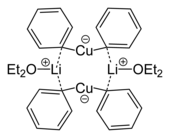
Cuprates have a role in organic synthesis. They are invariably Cu(I), although Cu(II) or even Cu(III) intermediates are invoked in some mechanisms. Organic cuprates often have the idealized formulas [CuR2]− and [CuR3]2−, where R is an alkyl or aryl. These reagents find use as nucleophilic alkylating reagents.[14]
See also
References
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ G. Brauer, ed. (1963). "Potassium Cuprate (III)". Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). NY: Academic Press. p. 1015.
- ^ Stricker, Marion; Linder, Thomas; Oelkers, Benjamin; Sundermeyer, Jörg (2010). "Cu(i)/(ii) based catalytic ionic liquids, their metallo-laminate solid state structures and catalytic activities in oxidative methanol carbonylation". Green Chemistry. 12 (9): 1589. doi:10.1039/c003948a.
- ^ Kroeker, Scott; Wasylishen, Roderick E. (1999). "Article". Canadian Journal of Chemistry. 77 (11): 1962–1972. doi:10.1139/v99-181.
- ^ Bowmaker, Graham A.; Hartl, Hans; Urban, Victoria (2000). "Crystal Structures and Vibrational Spectroscopy of [NBu4][Cu(CN)X] (X = Br, I) and [NBu4][Cu3(CN)4]·CH3CN". Inorganic Chemistry. 39 (20): 4548–4554. doi:10.1021/ic000399s.
- ^ Willett, Roger D.; Butcher, Robert E.; Landee, Christopher P.; Twamley, Brendan (2006). "Two Halide Exchange in Copper(II) Halide Dimers: (4,4′-Bipyridinium)Cu2Cl6−x BRX". Polyhedron. 25 (10): 2093–2100. doi:10.1016/j.poly.2006.01.005.
- ^ Hasselgren, Catrin; Jagner, Susan; Dance, Ian (2002). "Three-Coordinate [CuIIX3]− (X=Cl, Br), Trapped in a Molecular Crystal". Chemistry - A European Journal. 8 (6): 1269–1278. doi:10.1002/1521-3765(20020315)8:6<1269::AID-CHEM1269>3.0.CO;2-9.
- ^ Mahoui, A.; Lapasset, J.; Moret, J.; Saint Grégoire, P. (1996). "Tetraethylammonium Tetramethylammonium Tetrachlorocuprate(II), [(C2H5)4N][(CH3)4N][CuCl4]". Acta Crystallographica Section C. 52 (11): 2674–2676. doi:10.1107/S0108270196009031.
- ^ "Reversible Extrusion and Uptake of HCl Molecules by Crystalline Solids Involving Coordination Bond Cleavage and Formation". J. Am. Chem. Soc. 128 (30): 9584–9585. 2006. doi:10.1021/ja0625733. PMID 16866484.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Kelley, A.; Nalla, S.; Bond, M. R. (2015). "The square-planar to flattened-tetrahedral CuX42- (X = Cl, Br) structural phase transition in 1,2,6-trimethylpyridinium salts". Acta. Cryst. B. 71 (Pt 1): 48–60. doi:10.1107/S205252061402664X. PMID 25643715.
- ^ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic Chemistry. Academic Press. pp. 1252–1264. ISBN 0-12-352651-5.
- ^ "Sodium Tetrahydroxocuprate (II) in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1015.
- ^ Lorenzen, Nis Peter; Weiss, Erwin (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt2)}(CuPh2)]2". Angewandte Chemie International Edition in English. 29 (3): 300. doi:10.1002/anie.199003001.
- ^ Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1-891389-04-1.
