Permanganate

| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Permanganate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MnO− 4 | |
| Molar mass | 118.934 g·mol−1 |
| Conjugate acid | Permanganic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
A permanganate is the general name for a chemical compound containing the manganate(VII) ion, (MnO−
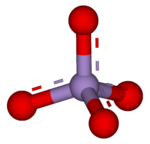
4). Because manganese is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry.[1] Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized. For example, trichloroethane (C2H3Cl3) is oxidized by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).[2]
- 8MnO−
4 + 3C
2H
3Cl
3 → 6CO
2 + 8MnO
2 + H+
+ 4H
2O + 9Cl−
In an acidic solution, permanganate(VII) is reduced to the pale pink +2 oxidation state of the manganese(II) (Mn2+) ion.
- 8 H+
+ MnO−
4 + 5 e− → Mn2+ + 4 H2O
In a strongly basic solution, permanganate(VII) is reduced to the green +6 oxidation state of the manganate ion, MnO2−
4.
- MnO−
4 + e− → MnO2−
4
In a neutral medium, however, it gets reduced to the brown +4 oxidation state of manganese dioxide MnO2.
- 2 H2O + MnO−
4 + 3 e− → MnO2 + 4 OH−
Production
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide:
- 2 MnCl2 + 5 NaClO + 6 NaOH → 2 NaMnO4 + 9 NaCl + 3 H2O
- 2 MnSO4 + 5 PbO2 + 3 H2SO4 → 2 HMnO4 + 5 PbSO4 + 2 H2O
It may also be produced by the disproportionation of manganates, with manganese dioxide as a side-product:
- 3 Na2MnO4 + 2 H2O → 2 NaMnO4 + MnO2 + 4 NaOH
They are produced commercially by electrolysis or air oxidation of alkaline solutions of manganate salts (MnO2−
4).[3]

Properties

Permanganates(VII) are salts of permanganic acid. They have a deep purple colour, due to a charge transfer transition. Permanganate(VII) is a strong oxidizer, and similar to perchlorate. It is therefore in common use in qualitative analysis that involves redox reactions (permanganometry). According to theory, permanganate is strong enough to oxidize water, but this does not actually happen to any extent. Besides this, it is stable.
It is a useful reagent, though with organic compounds, not very selective. Potassium permanganate is used as a disinfectant and water treatment additive in aquaculture.[4]
Manganates(VII) are not very stable thermally. For instance, potassium permanganate decomposes at 230 °C to potassium manganate and manganese dioxide, releasing oxygen gas:
- 2 KMnO4 → K2MnO4 + MnO2 + O2
A permanganate can oxidize an amine to a nitro compound,[5][6] an alcohol to a ketone,[7] an aldehyde to a carboxylic acid,[8][9] a terminal alkene to a carboxylic acid,[10] oxalic acid to carbon dioxide,[11] and an alkene to a diol.[12] This list is not exhaustive.
In alkene oxidations one intermediate is a cyclic Mn(V) species[citation needed]:
Compounds
- Ammonium permanganate, NH4MnO4
- Calcium permanganate, Ca(MnO4)2
- Potassium permanganate, KMnO4
- Sodium permanganate, NaMnO4
- Silver permanganate, AgMnO4
See also
- Perchlorate, a similar ion with a chlorine(VII) center
- Chromate, which is isoelectronic with permanganate
- Pertechnetate
References
- ^ Sukalyan Dash, Sabita Patel & Bijay K. Mishra (2009). "Oxidation by permanganate: synthetic and mechanistic aspects". Tetrahedron. 65 (4): 707–739. doi:10.1016/j.tet.2008.10.038.
- ^ http://geocleanse.com/permanaganate.asp
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Carlos A. Murillo; Manfred Bochmann (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc. p. 770. ISBN 978-0471199571.
- ^ Syndel. "Potassium Permanganate Sodium Disinfectant".
- ^ A. Calder, A. R. Forrester1, and S. P. Hepburn (1972). "2-methyl-2-nitrosopropane and its dimer". Organic Syntheses. 6: 803
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link); Collected Volumes, vol. 52, p. 77. - ^ Nathan Kornblum and Willard J. Jones (1963). "4-nitro-2,2,4-trimethylpentane". Organic Syntheses. 5: 845; Collected Volumes, vol. 43, p. 87.
- ^ J. W. Cornforth (1951). "Ethyl pyruvate". Organic Syntheses. 4: 467; Collected Volumes, vol. 31, p. 59.
- ^ R. L. Shriner and E. C. Kleiderer (1930). "Piperonylic acid". Organic Syntheses. 2: 538; Collected Volumes, vol. 10, p. 82.
- ^ John R. Ruhoff (1936). "n-heptanoic acid". Organic Syntheses. 2: 315; Collected Volumes, vol. 16, p. 39.
- ^ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1981). "Carboxylic acids from the oxidation of terminal alkenes by permanganate: nonadecanoic acid". Organic Syntheses. 7: 397
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 60, p. 11. - ^ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A. 108 (50): 11026. Bibcode:2004JPCA..10811026K. doi:10.1021/jp047061u.
- ^ E. J. Witzemann, Wm. Lloyd Evans, Henry Hass, and E. F. Schroeder (1931). "dl-glyceraldehyde ethyl acetal". Organic Syntheses. 2: 307
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 11, p. 52.

