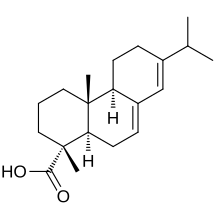
Abietic acid
| |
| |
| Names | |
|---|---|
| IUPAC name
Abieta-7,13-dien-18-oic acid
| |
| Other names
(1R,4aR,4bR,10aR)-7-Isopropyl-1,4a-dimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene-1-carboxylic acid; Abietinic acid; Sylvic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.436 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.458 g·mol−1 |
| Appearance | yellow resinous powder, crystals or chunks. Monoclinic plates (from EtOH/water). |
| Melting point | 173.5 °C (344.3 °F; 446.6 K) |
| Boiling point | 250 °C at 9 mmHg |
| Very soluble in acetone, petroleum ether, Et2O, EtOH. | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Abietic acid (also known as abietinic acid or sylvic acid), a resin acid, is the primary irritant in pine wood and resin, isolated from rosin (via isomerization) and is the most abundant of several closely-related organic acids that constitute most of rosin, the solid portion of the oleoresin of coniferous trees. Its ester is called an abietate.
Abietic acid is a weak contact allergen; however, compounds resulting of its oxidation by air elicit stronger response. It is soluble in alcohols, acetone, and ethers.
Commercial abietic acid is usually a glassy or partly crystalline yellowish solid that melts at temperatures as low as 85°C (185°F). It belongs to the diterpene group of organic compounds (compounds derived from four isoprene units). It is used in lacquers, varnishes, and soaps, and for the analysis of resins and the preparation of metal resinates. It is listed in the Toxic Substances Control Act inventory.
Rosin has been used for centuries for caulking ships. It is also rubbed on the bows of musical instruments to make them less slippery. In modern times methods have been developed for improving the properties of the rosin acids, which are otherwise soft, tacky, and low-melting and subject to rapid deterioration by oxidation in air. Stability is greatly increased by heat treatment.
Rosin acids are converted into ester gum by reaction with controlled amounts of glycerol or other polyhydric alcohols. Ester gum has drying properties and is used in paints, varnishes, and lacquers.
It is found in Pinus insularis (Khasi Pine), Pinus kesiya Royle, Pinus strobus (Eastern White Pine),Pinus sylvestris(Scotch Pine) [2]
In vitro effects
The 50% ethanol extracts from Resina pini of Pinus sp. (Pinaceae) showed more potent inhibitory activity against testosterone 5α-reductase prepared from rat prostate than those from several medicinal plants used for the treatment of androgen-dependent diseases such as benign prostatic hyperplasia. The fraction responsible for this activity was purified, and the active constituent was isolated and identified as abietic acid which exhibited potent testosterone 5α-reductase inhibitory activity in vitro.[3]
References
- ^ "ABIETIC ACID - PubChem Public Chemical Database". pubchem.ncbi.nlm.nih.gov. Retrieved 2008-05-16.
- ^ Duke's data Base http://www.ars-grin.gov/cgi-bin/duke/highchem.pl
- ^ Seong-Soo Roh, Moon-Ki Park and Yong-ung Kim (2010). "Abietic Acid from Resina Pini of Pinus Species as a Testosterone 5α-Reductase Inhibitor". J. Health Sci. 56: 451–455. doi:10.1248/jhs.56.451.

