Agelasimine
Appearance
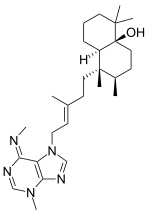
Agelasimines are a group of adenine-related bicyclic diterpenoids isolated from the orange sponge Agelas mauritania.[1] Their chemical structures are closely related to the agelasines.
Both groups of compounds display a range of biological activities, such as cytotoxicity, inhibition of adenosine transfer into rabbit erythrocytes (red blood cells), Ca2+ channel antagonistic action, α1 adrenergic blockade and others.[2][3]
Both compounds have been reproduced in the laboratory by organic synthesis.
References
[edit]- ^ Ohba, Masashi; Iizuka, Kazuaki; Ishibashi, Hiroyuki; Fujii, Tozo (December 1997). "Syntheses and absolute configurations of the marine sponge purines (+)-agelasimine-A and (+)-agelasimine-B". Tetrahedron. 53 (50): 16977–16986. doi:10.1016/S0040-4020(97)10120-X.
- ^ Fathi-Afshar, R.; Allen, T. M. (January 1988). "Biologically active metabolites from". Canadian Journal of Chemistry. 66 (1): 45–50. doi:10.1139/v88-006.
- ^ Fathi-Afshar, R.; Allen, T. M.; Krueger, C. A.; Cook, D. A.; Clanachan, A. S.; Vriend, R.; Baer, H. P.; Cass, C. E. (April 1989). "Some pharmacological activities of novel adenine-related compounds isolated from a marine sponge". Canadian Journal of Physiology and Pharmacology. 67 (4): 276–281. doi:10.1139/y89-045.
