Allotropes of iron


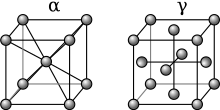
Iron represents perhaps the best-known example for allotropy in a metal. At atmospheric pressure, three allotropic forms of iron exist: alpha iron (α), gamma iron (γ) (also known as austenite), and delta iron (δ). At very high pressure, a fourth form exists, called epsilon iron (ε) hexaferrum. Some controversial experimental evidence exists for another high-pressure form that is stable at very high pressures and temperatures.[1]
The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure.[2][3][4] The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements.
Standard pressure allotropes
Delta iron (δ-Fe)
As molten iron cools down, it solidifies at 1,538 °C (2,800 °F) into its δ allotrope, which has a body-centered cubic (BCC) crystal structure.[5] δ-iron can dissolve as much as 0.08% of carbon by mass at 1,475°C.
Gamma iron / Austenite(γ-Fe)
As the iron cools further to 1,394 °C its crystal structure changes to a face centered cubic (FCC) crystalline structure. In this form it is called gamma iron (γ-Fe) or Austenite. γ-iron can dissolve considerably more carbon (as much as 2.04% by mass at 1,146 °C). This γ form of carbon saturation is exhibited in stainless steel.
Beta iron (β-Fe)
Beta iron (β-Fe) are obsolete terms for the paramagnetic allotrope of iron.[6][7] The primary phase of low-carbon or mild steel and most cast irons at room temperature is ferromagnetic α-Fe. As iron or steel is heated above the critical temperature A2 or Curie temperature of 771 °C (1044K or 1420 °F),[8] the random thermal agitation of the atoms exceeds the oriented magnetic moment of the unpaired electron spins.[9] The A2 forms the low-temperature boundary of the beta iron field in the phase diagram in Figure 1. β-Fe is crystallographically identical to α-Fe, except for magnetic domains and the expanded body-centered cubic lattice parameter as a function of temperature, and is therefore of only minor importance in steel heat treating. For this reason, the beta "phase" is not usually considered a distinct phase but merely the high-temperature end of the alpha phase field.
Similarly, the A2 is of only minor importance compared to the A1 (eutectoid), A3 and Acm critical temperatures. The Acm, where austenite is in equilibrium with cementite + γ-Fe, is beyond the right edge in Fig. 1. The α + γ phase field is, technically, the β + γ field above the A2. The beta designation maintains continuity of the Greek-letter progression of phases in iron and steel: α-Fe, β-Fe, austenite (γ-Fe), high-temperature δ-Fe, and high-pressure hexaferrum (ε-Fe).

A2 critical temperature and induction heating
β-Fe and the A2 critical temperature are important in induction heating of steel, such as for surface-hardening heat treatments. Steel is typically austenitized at 900–1000 °C before it is quenched and tempered. The high-frequency alternating magnetic field of induction heating heats the steel by two mechanisms below the Curie temperature: resistance or Joule (I2R) heating and ferromagnetic hysteresis losses. Above the A2, the hysteresis mechanism disappears and the required amount of energy per degree of temperature increase is substantially larger than below A2. Load-matching circuits may be needed to vary the impedance in the induction power source to compensate for the change.[10]
Alpha iron (α-Fe)
Below 912 °C (1,674 °F) iron again adopts the BCC structure characteristic of α-iron, also called ferrite. The substance assumes a paramagnetic property. Carbon dissolves poorly in α-iron: no more than 0.021% by mass at 723 °C.
As it cools to 770 °C (1,418 °F), the Curie point (TC), the iron is a fairly soft metal and becomes ferromagnetic. As iron passes below the Curie temperature, no structural change occurs, but the magnetic properties as the magnetic domains become aligned. This form of iron is stable form at room temperature. α-Fe can be subjected to pressures up to ca. 15 GPa before transforming into a high-pressure form termed ε-iron, which crystallizes in a hexagonal close-packed (hcp) structure.
α-Fe is a component of steel and cast iron, conferring Ferromagnetism.[11][12] It has a hardness of approximately 80 Brinell.[13][14] the maximum solubility is about 0.02 wt% at 727 °C (1,341 °F) and 0.001% carbon at 0 °C (32 °F).[15] When it dissolves in iron, carbon atoms occupy interstitial "holes". Being about twice the diameter of the tetrahedral hole, the carbon introduces a strong local strain field.
Mild steel (carbon steel with up to about 0.2 wt% C) consist mostly of α-Fe and increasing amounts of cementite (Fe3C, an iron carbide). The mixture adopts a laminar structure called pearlite. Since bainite and pearlite each contain α-Fe as a component, any iron-carbon alloy will contain some amount of α-Fe if it is allowed to reach equilibrium at room temperature. The amount of α-Fe depends on the cooling process.

High pressure allotropes
Epsilon iron / Hexaferrum (ε-Fe)
At pressures above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into a hexagonal close-packed (hcp) structure, which is also known as ε-iron or hexaferrum;[16] the higher-temperature γ-phase also changes into ε-iron, but does so at a higher pressure. Antiferromagnetism in alloys of epsilon-Fe with Mn, Os and Ru has been observed.[17]
Experimental high temperature and pressure
An alternate stable form, if it exists, may appear at pressures of at least 50 GPa and temperatures of at least 1,500 K; it has been thought to have an orthorhombic or a double hcp structure.[1] as of December 2011, recent and ongoing experiments are being conducted on high-pressure and Superdense carbon allotropes.
See also
- Superdense carbon allotropes
- Allotropy
- Austenite
- Curie point
- Eutectic system
- Tempering (metallurgy)
- ((Nature of crystal structure))
References
- ^ a b Boehler, Reinhard (2000). "High-pressure experiments and the phase diagram of lower mantle and core materials". Review of Geophysics. 38 (2). American Geophysical Union: 221–245. Bibcode:2000RvGeo..38..221B. doi:10.1029/1998RG000053.
- ^ Cohen, Ronald; Stixrude, Lars. "Crystal at the Center of the Earth". Archived from the original on 5 February 2007. Retrieved 2007-02-05.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Stixrude, Lars; Cohen, R.E. (March 1995). "High-Pressure Elasticity of Iron and Anisotropy of Earth's Inner Core". Science. 267 (5206): 1972–5. Bibcode:1995Sci...267.1972S. doi:10.1126/science.267.5206.1972. PMID 17770110.
- ^ "What is at the centre of the Earth?". BBC News. 31 August 2011.
- ^ Lyman, Taylor, ed. (1973). Metallography, Structures and Phase Diagrams. Metals Handbook. Vol. 8 (8th ed.). Metals Park, Ohio: ASM International. OCLC 490375371.
- ^ D. K. Bullens et al., Steel and Its Heat Treatment, Vol. I, Fourth Ed., J. Wiley & Sons Inc., 1938, p. 86.
- ^ Avner, S.H. (1974). Introduction to physical metallurgy (2nd ed.). McGraw-Hill. p. 225. ISBN 978-0-07-002499-1.
- ^ a b Alloy Phase Diagrams. ASM Handbook. Vol. 3. ASM International. 1992. pp. 2.210, 4.9. ISBN 0-87170-381-5.
- ^ Cullity, B.D.; Graham, C.D. (2009). Introduction to Magnetic Materials (2nd ed.). IEEE. p. 91. ISBN 978-0-471-47741-9.
- ^ Semiatin, S.L.; Stutz, D.E. (1986). Induction Heat Treatment of Steel. ASM International. pp. 95–98. ISBN 0-87170-211-8.
- ^ Maranian, Peter (2009), Reducing Brittle and Fatigue Failures in Steel Structures, New York: American Society of Civil Engineers, ISBN 978-0-7844-1067-7.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Structure of plain steel, retrieved 2008-10-21.
- ^ Alvarenga HD, Van de Putte T, Van Steenberge N, Sietsma J, Terryn H (January 2015). "Influence of Carbide Morphology and Microstructure on the Kinetics of Superficial Decarburization of C-Mn Steels". Metal Mater Trans A. 46 (1): 123–133. Bibcode:2015MMTA...46..123A. doi:10.1007/s11661-014-2600-y.
- ^ Smith & Hashemi 2006, p. 363.
- ^ Mathon O; Baudelet F; Itié JP; Polian A; d'Astuto M; Chervin JC; Pascarelli S. (14 December 2004). "Dynamics of the magnetic and structural alpha-epsilon phase transition in iron". Physical Review Letters. 93 (25): 255503. arXiv:cond-mat/0405439. Bibcode:2004PhRvL..93y5503M. doi:10.1103/PhysRevLett.93.255503. PMID 15697906.
- ^ G. C. Fletcher; R. P. Addis (November 1974). "The magnetic state of the phase of iron" (PDF). Journal of Physics F: Metal Physics. Vol. 4, no. 11. p. 1954. Bibcode:1974JPhF....4.1951F. doi:10.1088/0305-4608/4/11/020. Retrieved December 30, 2011.
