Atomic clock

An atomic clock is a type of clock that uses an atomic resonance frequency standard to feed its counter. Early atomic clocks were masers with attached equipment. Today's best atomic frequency standards (or clocks) are based on more advanced physics involving cold atoms and atomic fountains. National standards agencies maintain an accuracy of 10-9 seconds per day, and a precision equal to the frequency of the radio transmitter pumping the maser. The clocks maintain a continuous and stable time scale, International Atomic Time (TAI). For civil time, another time scale is disseminated, Coordinated Universal Time (UTC). UTC is derived from TAI, but synchronized with the passing of day and night based on astronomical observations.
The first atomic clock was built in 1949 at the U.S. National Bureau of Standards (NBS). The first accurate atomic clock, based on the transition of the caesium-133 atom, was built by Louis Essen in 1955 at the National Physical Laboratory in the UK. This led to the internationally agreed definition of the second being based on atomic time.
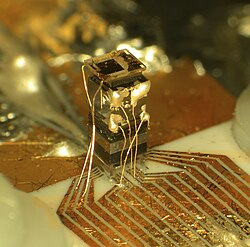
In August 2004, NIST scientists demonstrated a chip-scaled atomic clock. According to the researchers, the clock was believed to be one hundredth the size of any other. It was also claimed that it requires just 75 mW, making it suitable for battery-driven applications.
Modern radio clocks are referenced to atomic clocks, and provide a way of getting high-quality atomic-derived time over a wide area using inexpensive equipment; however, radio clocks are not appropriate for high-precision scientific work.
How they work
Frequency reference masers uses glowing chambers of ionized gas, most often caesium, because caesium is the element used in the official international definition of the second.
Since 1967, the International System of Units (SI) has defined the second as 9,192,631,770 cycles of the radiation which corresponds to the transition between two energy levels of the ground state of the Caesium-133 atom. This definition makes the caesium oscillator (often called an atomic clock) the primary standard for time and frequency measurements (see caesium standard). Other physical quantities, like the volt and metre, rely on the definition of the second as part of their own definitions.
The core of the atomic clock is a microwave cavity containing the ionized gas, a tunable microwave radio oscillator, and a feedback loop which is used to adjust the oscillator to the exact frequency of the absorption characteristic defined by the behavior of the individual atoms.
The microwave oscillator fills the chamber with a standing wave of radio waves. When the radio frequency matches the hyperfine transition frequency of caesium, electrons in the caesium atoms are able to absorb the radio waves and move between two energy states. Only those atoms that have changed states are allowed to impinge on a detector. When the incidence of detected atoms decreases because the frequency of the microwave oscillator has drifted from the true resonance frequency, the frequency of the oscillator is corrected.
This adjustment process is where most of the work and complexity of the clock lies. The adjustment tries to correct for unwanted side-effects, such as frequencies from other electron transitions, temperature changes, and the "spreading" in frequencies caused by ensemble effects. One way of doing this is to sweep the microwave oscillator's frequency across a narrow range to generate a modulated signal at the detector. The detector's signal can then be demodulated to apply feedback to control long-term drift in the radio frequency. In this way, the quantum-mechanical properties of the atomic transition frequency of the caesium can be used to tune the microwave oscillator to the same frequency (except for a small amount of experimental error). When a clock is first turned on, it takes a while for the oscillator to stablize.
In practice, the feedback and monitoring mechanism is much more complex than described above.

A number of other atomic clock schemes are in use for other purposes. Rubidium clocks are prized for their low cost, small size (commercial standards are as small as 400 cm3), and short term stability. They are used in many commercial, portable and aerospace applications. Hydrogen masers (often manufactured in Russia) have superior short term stability to other standards, but lower long term accuracy.
Often, one standard is used to fix another. For example, some commercial applications use a Rubidium standard slaved to a GPS receiver. This achieves excellent short term accuracy, with long term accuracy equal to (and traceable to) the U.S. national time standards.
The lifetime of a standard is an important practical issue. Modern Rubidium standard tubes last more than ten years, and can cost as little as $50 US. Caesium reference tubes suitable for national standards currently last about seven years, and cost about $35,000 US. Hydrogen standards have an unlimited lifetime.
Research
Most research focuses on way to make the clocks smaller, cheaper, more accurate, and more reliable. These goals usually conflict.
A major push in current research on atomic clocks is the development of clocks based on optical transitions rather than microwave transitions. The higher frequency of optical transitions combined with highly stabilized laser systems allows for greater frequency stability. Before the demonstration of the self-referenced femtosecond frequency comb in 2000, there was no straightforward method for making an absolute measurement of optical frequencies. With the refinement of the frequency comb these measurements have become much more accessible and numerous optical clock systems are now being developed around the world. The two primary technologies under consideration for use in optical frequency standards are single ions isolated in an ion trap and neutral atoms trapped in an optical lattice. These two techniques allow the atoms or ions to be highly isolated from external perturbations, thus producing an extremely stable frequency reference. Atomic systems under consideration include but are not limited to Al+, Hg+, Sr, and Yb.
See also
- Network Time Protocol
- NIST-F1
- Optical Atomic Clock [1]
- Radio clock
- Second
- Télé Distribution Francaise
External links
- PTB Braunschweig, Germany - with link in English language
- National Physical Laboratory (UK) time website
- NIST Internet Time Service (ITS): Set Your Computer Clock Via the Internet
- NIST press release about chip-scaled atomic clock
- NIST website
- Web pages on atomic clocks by The Science Museum (London)
