Aziridine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Aziridine
| |||
| Other names
Azacyclopropane, Ethylene imine, Aminoethylene, Azirane, Dimethyleneimine, Dimethylenimine, Ethylimine
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102380 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.268 | ||
| EC Number |
| ||
| 616 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1185 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5N | |||
| Molar mass | 43.069 g·mol−1 | ||
| Appearance | Clear colorless oily liquid[1] | ||
| Odor | ammonia-like[2] | ||
| Density | 0.8321 g/mL 20 °C[3] | ||
| Melting point | −77.9 °C (−108.2 °F; 195.2 K) | ||
| Boiling point | 56 °C (133 °F; 329 K) | ||
| miscible | |||
| Vapor pressure | 160 mmHg (20° C)[2] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable and toxic | ||
| GHS labelling: | |||
    
| |||
| Danger | |||
| H225, H300, H310, H314, H330, H340, H350, H411 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+P310, P301+P330+P331, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P320, P321, P322, P330, P361, P363, P370+P378, P391, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 322 °C (612 °F; 595 K) | |||
| Explosive limits | 3.6–46% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
250 ppm (rat, 1 hr) 250 ppm (guinea pig, 1 hr) 62 ppm (rat, 4 hr) 223 ppm (mouse, 2 hr) 56 ppm (rat, 2 hr) 2236 ppm (mouse, 10 min)[4] | ||
LCLo (lowest published)
|
25 ppm (guinea pig, 8 hr) 56 ppm (rabbit, 2 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
OSHA-Regulated Carcinogen[2] | ||
REL (Recommended)
|
Ca[2] | ||
IDLH (Immediate danger)
|
Ca [100 ppm][2] | ||
| Related compounds | |||
Related heterocycles
|
Borirane Ethylene oxide Thiirane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group (-NH-) and two methylene bridges (-CH
2-).[5][6] The parent compound is aziridine (or ethylene imine), with molecular formula C
2H
5N.
Structure
The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrogen free electron pair. Angle strain in aziridine also increases the barrier to nitrogen inversion. This barrier height permits the isolation of separate invertomers, for example the cis and trans invertomers of N-chloro-2-methylaziridine.
Synthesis
There are several syntheses of aziridines (aziridination).
Cyclization of haloamines and amino alcohols
An amine functional group displaces the adjacent halide in an intramolecular nucleophilic substitution reaction to generate an aziridine. Amino alcohols have the same reactivity, but the hydroxy group must first be converted into a good leaving group. The cyclization of an amino alcohol is called a Wenker synthesis (1935), and that of a haloamine the Gabriel ethylenimine method (1888).[citation needed]
Nitrene addition
Nitrene addition to alkenes is a well-established method for the synthesis of aziridines. Photolysis or thermolysis of azides are good ways to generate nitrenes. Nitrenes can also be prepared in situ from iodosobenzene diacetate and sulfonamides, or the ethoxycarbonylnitrene from the N-sulfonyloxy precursor.[7]

Triazoline decomposition
Thermal treatment or photolysis of triazolines expels nitrogen, producing an aziridine. Triazolines can be generated by cycloaddition of alkenes with an azide.
From epoxides
One method involves the ring-opening reaction of an epoxide with sodium azide, followed by organic reduction of the azide with triphenylphosphine accompanied by expulsion of nitrogen gas:[8]

The other method involves the ring-opening reaction of an epoxide with amines, followed by ring closing with the Mitsunobu reaction.[9]
From oximes
The Hoch-Campbell ethylenimine (Aziridine) synthesis is the reaction of certain oximes with Grignard reagents:[10][11][12][13]

From alkenes using DPH
In 2014, a new method was described to produce aziridines by reacting a mono-, di-, tri- or tetra- substituted alkene (olefin) with O-(2,4-dinitrophenyl)hydroxylamine (DPH) via homogeneous rhodium catalysis, alone. This method is operationally simple (i.e., one-pot) with excellent yield.
Alkene + DPH Aziridine
For instance, Ph-Aziridine-Me can be synthesyzed by this method and then converted by ring opening reaction to (D)-amphetamine and (L)-amphetamine (the two active ingredients in Adderall).[14]
Reactions
Nucleophilic ring opening
Aziridines are reactive substrates in ring-opening reactions with many nucleophiles due to their ring strain. Alcoholysis and aminolysis are basically the reverse reactions of the cyclizations. Carbon nucleophiles such as organolithium reagents and organocuprates are also effective.
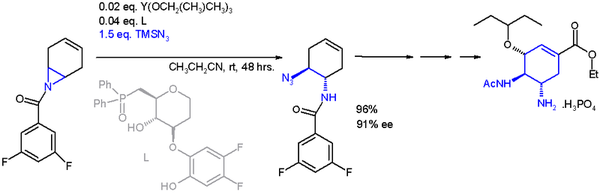
One application of a ring-opening reaction in asymmetric synthesis is that of trimethylsilylazide TMSN
3 with an asymmetric ligand[15] in scheme 2[16] in an organic synthesis of oseltamivir:
1,3-dipole formation
Certain N-substituted azirines with electron withdrawing groups on both carbons form azomethine ylides in an electrocyclic thermal or photochemical ring-opening reaction.[17][18] These ylides can be trapped with a suitable dipolarophile in a 1,3-dipolar cycloaddition.[19]
When the N-substituent is an electron-withdrawing group such as a tosyl group, the carbon-nitrogen bond breaks, forming another zwitterion TsN−
–CH
2–CH+
2–R[20]
This reaction type requires a Lewis acid catalyst such as boron trifluoride. In this way 2-phenyl-N-tosylaziridine reacts with alkynes, nitriles, ketones and alkenes. Certain 1,4-dipoles form from azetidines.
Other
N-unsubstituted aziridines can be opened with olefins in the presence of strong Lewis acid B(C
6F
5)
3.[21]
Human toxicology
The toxicology of a particular aziridine compound depends on its structure and activity, although sharing the general characteristics of aziridines. As electrophiles, aziridines are subject to attack and ring-opening by endogenous nucleophiles such as nitrogenous bases in DNA base pairs, resulting in potential mutagenicity.[22][23][24]
Exposure
Inhalation and direct contact are exposure routes. Some reports note that the use of gloves has not prevented permeation of aziridine. It is therefore important that users check the breakthrough permeation times for gloves, and pay scrupulous attention to avoiding contamination when degloving. Workers handling azidrine are expected to be provided with, and required to wear and use, a half-mask filter-type respirator for dusts, mists and fumes.[25]
There is relatively little human exposure data on aziridine. This is because it is considered extremely dangerous. In industrial settings, class A pressure suits are preferred when exposure is possible.
Carcinogenicity
The International Agency for Research on Cancer (IARC) has reviewed aziridine compounds and classified them as possibly carcinogenic to humans (IARC Group 2B).[26] In making the overall evaluation, the IARC Working Group took into consideration that aziridine is a direct-acting alkylating agent which is mutagenic in a wide range of test systems and forms DNA adducts that are promutagenic.
Irritancy
Aziridines are irritants of mucosal surfaces including eyes, nose, respiratory tract and skin.
Sensitization
Aziridine rapidly penetrates skin on contact.
Skin sensitizer — causing allergic contact dermatitis and urticaria.
Respiratory sensitiser — causing occupational asthma
See also
- Binary ethylenimine, a dimeric form of aziridine
References
- ^ "Aziridine" (PDF). Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 71. 1999.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0274". National Institute for Occupational Safety and Health (NIOSH).
- ^ Weast, Robert C.; et al. (1978). CRC Handbook of Chemistry and Physics (59th ed.). West Palm Beach, FL: CRC Press. ISBN 0-8493-0549-8.
- ^ a b "Ethyleneimine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Gilchrist, T.L. Heterocyclic chemistry. ISBN 0-582-01421-2.
- ^ Epoxides and aziridines – A mini review Albert Padwaa and S. Shaun Murphreeb Arkivoc (JC-1522R) pp. 6–33 Online article
- ^ M. Antonietta Loreto; Lucio Pellacani; Paolo A. Tardella; Elena Toniato (1984). "Addition reactions of ethoxycarbonylnitrene and ethoxycarbonylnitrenium ion to allylic ethers". Tetrahedron Letters. 25 (38): 4271–4. doi:10.1016/S0040-4039(01)81414-3.
- ^ Ryan Hili; Andrei K. Yudin (2006). "Readily Available Unprotected Amino Aldehydes". J. Am. Chem. Soc. 128 (46): 14772–3. doi:10.1021/ja065898s.
- ^ B. Pulipaka; Stephen C. Bergmeier (2008). "Synthesis of Hexahydro-1 H -benzo[ c ]chromen-1-amines via the Intramolecular Ring-Opening Reof Aziridines by π-Nucleophiles". Synthesis. 2008 (9): 1420–30. doi:10.1055/s-2008-1072561.
- ^ Hoch, Compt. rend., 196, 1865 (1934); (a), ibid., aOS, 799 (1936); (e), ibid., 204, 358 (1937).
- ^ Kenneth N. Campbell; James F. Mckenna (1939). "The action of Grignard reagents on oximes. i. The action of phenylmagnesium bromide on mixed ketoximes". J. Org. Chem. 4 (2): 198–205. doi:10.1021/jo01214a012.
- ^ Kenneth N. Campbell; Barbara Knapp Campbell; Elmer Paul Chaput (1943). "The reaction of Grignard reagents with oximes. ii. The action of aryl grignard reagents with mixed ketoximes". J. Org. Chem. 8 (1): 99–102. doi:10.1021/jo01189a015.
- ^ Kenneth N. Campbell; Barbara K. Campbell; James F. Mckenna; Elmer Paul Chaput (1943). "The action of Grignard reagents on oximes. iii. The mechanism of the action of arylmagnesium halides on mixed ketoximes. A new synthesis of ethyleneimines". J. Org. Chem. 8: 103–9. doi:10.1021/jo01189a016.
- ^ Jat, Jawahar L.; Paudyal, Mahesh P.; Gao, Hongyin; Xu, Qing-Long; Yousufuddin, Muhammed; Devarajan, Deepa; Ess, Daniel H.; Kürti, László; Falck, John R. (2014-01-03). "Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins". Science. 343 (6166): 61–65. doi:10.1126/science.1245727. ISSN 0036-8075. PMC 4175444. PMID 24385626.
- ^ Yuhei Fukuta; Tsuyoshi Mita; Nobuhisa Fukuda; Motomu Kanai; Masakatsu Shibasaki (2006). "De Novo Synthesis of Tamiflu via a Catalytic Asymmetric Ring-Opening of meso-Aziridines with TMSN3". J. Am. Chem. Soc. 128 (19): 6312–3. doi:10.1021/ja061696k.
- ^ The catalyst is based on yttrium with three isopropyloxy substituents and the ligand a phosphine oxide (Ph = phenyl), with 91% enantiomeric excess (ee)
- ^ Harold W. Heine; Richard Peavy (1965). "Aziridines XI. Reaction of 1,2,3-triphenylaziridine with diethylacetylene dicarboxylate and maleic anhydride". Tetrahedron Letters. 6 (35): 3123–6. doi:10.1016/S0040-4039(01)89232-7.
- ^ Albert Padwa; Lewis Hamilton (1965). "Reactions of aziridines with dimethylacetylene dicarboxylate". Tetrahedron Letters. 6 (48): 4363–7. doi:10.1016/S0040-4039(00)71101-4.
- ^ Philippe Dauban; Guillaume Malik (2009). "A Masked 1,3-Dipole Revealed from Aziridines". Angew. Chem. Int. Ed. 48 (48): 9026–9. doi:10.1002/anie.200904941. PMID 19882612.
- ^ Ioana Ungureanua; Cristian Bologab; Saïd Chayera; André Mann (16 July 1999). "Phenylaziridine as a 1,3-dipole. Application to the synthesis of functionalized pyrrolidines". Tetrahedron Letters. 40 (29): 5315–8. doi:10.1016/S0040-4039(99)01002-3.
- ^ Aravinda B. Pulipaka; Stephen C. Bergmeier (2008). "A Synthesis of 6-Azabicyclo[3.2.1]octanes. The Role of N-Substitution". J. Org. Chem. 73 (4): 1462–7. doi:10.1021/jo702444c.
- ^ Kanerva L, Keskinen H, Autio P, Estlander T, Tuppurainen M, Jolanki R (May 1995). "Occupational respiratory and skin sensitization caused by polyfunctional aziridine hardener". Clin Exp Allergy. 25 (5): 432–9. doi:10.1111/j.1365-2222.1995.tb01074.x. PMID 7553246.
- ^ Sartorelli P, Pistolesi P, Cioni F, Napoli R, Sisinni AG, Bellussi L, Passali GC, Cherubini Di Simplicio E, Flori L (2003). "Skin and respiratory allergic disease caused by polyfunctional aziridine". Med Lav. 94 (3): 285–95. PMID 12918320.
- ^ Mapp CE (2001). "Agents, old and new, causing occupational asthma". Occup. Environ. Med. 58 (5): 354–60. doi:10.1136/oem.58.5.354. PMC 1740131. PMID 11303086.
- ^ Appendix E - OSHA Respirator Requirements for Selected Chemicals
- ^ Some Aziridines, N-, S- and O-Mustards and Selenium (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 9. 1975. ISBN 92-832-1209-6.






