Baeyer–Drewsen indigo synthesis
The Baeyer–Drewson indigo synthesis (1882) is an organic reaction in which indigo is prepared from 2-nitrobenzaldehyde and acetone [1][2]

The reaction is classified as an aldol condensation. As a practical route to indigo, this method was displaced by routes from aniline.[3]
Mechanism
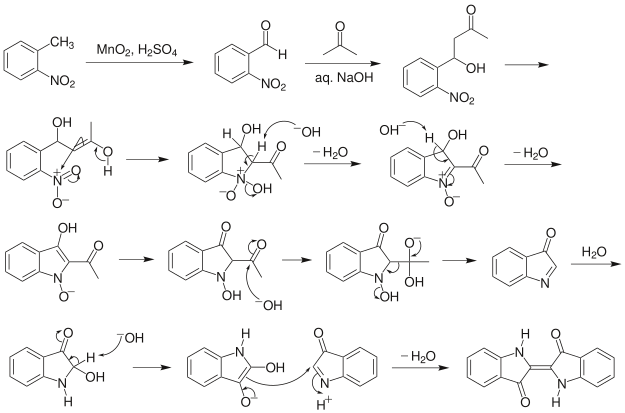
Note
In the English literature this reaction is usually called Baeyer–Drewson reaction, although the author of the original paper was called Drewsen.
References
- ^ Adolf Baeyer, Viggo Drewsen (1882). "Darstellung von Indigblau aus Orthonitrobenzaldehyd". Berichte der deutschen chemischen Gesellschaft. 15 (2): 2856–2864. doi:10.1002/cber.188201502274.
- ^ Helmut Schmidt (1997). "Indigo – 100 Jahre industrielle Synthese". Chemie in unserer Zeit. 31 (3): 121–128. doi:10.1002/ciuz.19970310304.
- ^ Elmar Steingruber "Indigo and Indigo Colorants" Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_149.pub2
