Bromous acid
Appearance
Template:Chembox Other
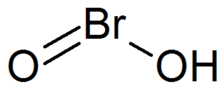
| |
| Names | |
|---|---|
| IUPAC names
hydroxy-λ3-bromanone
hydroxidooxidobromine bromous acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HBrO2 | |
| Molar mass | 112.911 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bromous acid with the formula HBrO2 has bromine in the +3 oxidation state. The salts of bromous acid are called bromites. The acid is not stable and only occurs as an intermediate, for example in the oxidation of hypobromites.[1]
Chemistry
Bromous acid can be produced by classical chemical or electrochemicals method via anodic oxidation.[citation needed]
- HBrO + HClO → HBrO2 + HCl
Also disproportioning of hypobromous acid will give bromous acid and hydrobromic acid.[citation needed]
- 2 HBrO → HBrO2 + HBr
Lastly, a synproportion reaction of bromic acid and hydrobromic acid gives bromous acid.[citation needed]
- 2 HBrO3 + HBr → 3 HBrO2
Compounds
Several bromites are stable and have been isolated. For example NaBrO2· 3H2O and Ba(BrO2)2·H2O.[1]
Use
Bromites can be used for the reduction of permanganates to manganates.[1]
- 2MnO−
4 + BrO−
2 + OH− → 2MnO2−
4 + BrO−
3 + H2O
