2,2',2''-Nitrilotriacetonitrile

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′,2′′-Nitrilotriacetonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.004 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6N4 | |
| Molar mass | 134.142 g·mol−1 |
| Structure[1] | |
| Pnma | |
| orthorhombic | |
a = 7.1085, b = 9.9320, c = 9.3869
| |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H302, H312, H315, H319, H335, H373 | |
| P260, P261, P264, P270, P271, P280, P301+P310, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P314, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrilotriacetonitrile (NTAN) is a precursor for nitrilotriacetic acid (NTA, a biodegradable complexing agent and building block for detergents), for tris(2-aminoethyl)amine (a tripodal tetradentate chelating agent known under the abbreviation tren) and for the epoxy resin crosslinker aminoethylpiperazine.
Production
[edit]The synthesis of nitrilotriacetonitrile is based on the basic building blocks ammonia, formaldehyde and hydrogen cyanide, which are reacted (via the triple cyanomethylation of the ammonia) in acidic aqueous medium in discontinuous or continuous processes.[2][3]
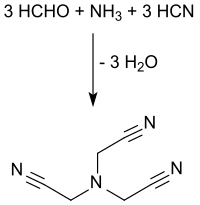
Ammonia is introduced as a gas, in form of hexamethylenetetramine[4] or as ammonium sulfate together with formaldehyde as aqueous solution (usually 37% by weight) at pH values <2 and treated with aqueous prussic acid solution or liquid hydrogen cyanide at temperatures around 100 °C. Prussic acid is used directly from the Andrussow process or the BMA process of Evonik Degussa[5] without pre-purification if necessary. When the mother liquors are returned, yields of more than 90% are achieved.
Problematic, particularly in the case of a continuous process, is the tendency of NTAN to precipitate at temperatures below 90 °C which can lead to clogging of tube reactors and conduits and thermal runaway of the reaction.[6]
Properties
[edit]Nitrilotriacetonitrile is a colorless and odorless solid which dissolves hardly in water but dissolves well in nitromethane and acetone.[7]
Use
[edit]Nitrilotriacetonitrile can be homopolymerized or copolymerized with iminodiacetonitrile in the melt in the presence of basic catalysts such as sodium methoxide to form dark-colored solid polymers which can be carbonized to form nitrogen-containing and electrically conductive polymers at temperatures above 1000 °C.[8] The products obtained have not found application as conductive polymers.
The hydrogenation of NTAN first converts a cyano group into an imino group which attacks a cyano group (which are adjacent and sterically suitable for forming a six-membered ring) rather than being further hydrogenated to the primary amino group. The end product of the catalytic hydrogenation of nitrilotriacetonitrile is therefore 1-(2-aminoethyl)piperazine.

If the catalytic hydrogenation of NTAN is carried out with e. g. Raney nickel in the presence of a large excess of ammonia, it gives tris(2-aminoethyl)amine.[9]

Tris(2-aminoethyl)amine is used as a tetrazident complexing agent (abbreviated as "tren"), which forms stable chelates, particularly with divalent and trivalent transition metal ions.[10]
Nitrilotriacetonitrile reacts with methanal at pH 9.5 to give 2,2-dihydroxymethyl-nitrilotriacetonitrile, which is hydrolyzed with sodium hydroxide solution at 100 °C to give the trisodium salt of 2-hydroxymethylserine-N,N-diacetic acid, from which the free acid can be obtained by acidification in 51% yield.[11]

The compound is suitable as a complexing agent for heavy metal ions or alkaline earth metal ions, as a stabilizer for bleaching agents (e.g. for sodium perborate, in solid detergent preparations) and as a builder in detergents for inhibiting the formation of incrustations in textiles during laundering.
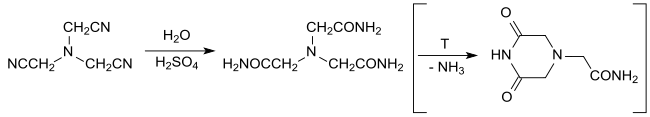
The hydrolysis of nitrilotriacetonitrile with water in concentrated sulfuric acid yields under gentle conditions practically quantitatively nitrilotriacetamide, which has been investigated as a neutral tetradentate ligand for metal complexation.[12] At elevated temperature, 3,5-dioxopiperazine-1-acetamide is formed by ring closure, which can be quantitatively converted into the nitrilotriacetamide after neutralization and heating with excess aqueous ammonia.[13][14]
Nitrilotriacetonitrile serves mainly as a raw material for the production of the biodegradable, but carcinogen suspected complexing agent nitrilotriacetic acid by acid or base-catalyzed[15][2] hydrolysis of the cyano groups.

Undesirable residual contents of cyanide ions in the hydrolyzate can be removed by post-treatment with oxidizing agents such as sodium hypochlorite at pH 8.[16]
References
[edit]- ^ CSD Entry: CIRWOR 2,2',2''-Nitrilotriacetonitrile
- ^ a b US 3337607, J.C. Wollensak, "Process for preparation of an amine nitrile", published 1967-08-22, assigned to Ethyl Corp.
- ^ US 3840581, H. Neumaier, W. Vogt, K. Sennewald, R. Schuller, G. Lenz, "Process for the manufacture of nitrilotriacetonitrile", published 1974-10-08, assigned to Knapsack AG
- ^ US 3061628, J.J. Singer Jr., M. Weisberg, "Process and preparation of amino nitriles and acetic acids", published 1962-10-30, assigned to Hampshire Chemical Corp.
- ^ EP 0102343, C.Y. Shen, "Process for producing nitrilotriacetonitrile", published 1986-02-26, assigned to Monsanto Co.
- ^ E. Fiedler (2016), "Emergency Runaway Reaction – What Precedes? What Follows?", Chem. Engineer. Transactions (CET), vol. 48, pp. 361–366, doi:10.3303/CET1648061, ISBN 978-88-95608-39-6
- ^ "Product Stewardship Summary, Chelates: NTAN" (PDF). akzonobel.com. Akzo Nobel Functional Chemicals. Archived from the original (PDF; 45.7 KB) on 2013-06-02. Retrieved 2017-03-20.
- ^ US 3578643, L.L. Wood, R.A. Hamilton, "New polymers from nitrilotriacetonitrile and iminodiacetonitrile", published 1971-05-11, assigned to W.R. Grace & Co.
- ^ US 3565957, S.B. Mirviss, D.J. Martin, E.D. Weil, "Hydrogenation of nitrilotriacetonitrile", published 1971-02-23, assigned to Stauffer Chemical Co.
- ^ G. Anderegg; V. Gramlich (1994), "1:1 Metal Complexes of Bivalent Cobalt, Nickel, Copper, Zink, and Cadmium with the Tripodal Ligand tris[2-(dimethylamino)ethyl]amine: Their stabilities and the X-ray crystal structure of its copper(II) complex sulfate", Helv. Chim. Acta, vol. 77, no. 3, pp. 685–690, doi:10.1002/hlca.19940770312
- ^ EP 0396999, A. Oftring, S. Birnbach, R. Bauer, C. Gousetis, W. Trieselt, "2-Methyl- und 2-Hydroxymethyl-serin-N,N-diessigsäure und ihre Derivate", published 1990-11-14, assigned to BASF AG
- ^ D.A. Smith; S. Sucheck; S. Cramer; D. Baker (1995), "Nitrilotriacetamide: Synthesis in concentrated sulfuric acid and stability in water", Synth. Commun., vol. 25, no. 24, pp. 4123–4132, doi:10.1080/00397919508011491
- ^ GB 1170399, "A process for preparing 3,5-dioxo-1-piperazineacetamide and nitrilotriacetic acid triamide", published 1969-11-12, assigned to W.R. Grace & Co.
- ^ D.A. Smith; S. Cramer; S. Sucheck; E. Skrzypzak-Jankun (1992), "Facile synthesis of substituted nitrilotriacetamides", Tetrahedron Lett., vol. 33, no. 50, pp. 7765–7768, doi:10.1016/0040-4039(93)88040-P
- ^ US 4547589, C.Y. Shen, "Hydrolysis of nitrilotriacetonitrile", published 1985-10-15, assigned to Monsanto Co.
- ^ US 8362298, O.M. Falana, A. Hikem, S.R. Kakadjian, F. Zamora, "Hydrolyzed nitrilotriacetonitrile compositions, nitrilotriacetonitrile hydrolysis formulations and methods for making and using same", published 2013-01-29, assigned to Clearwater International, LLC
