Carboxylate


A carboxylate is a salt or ester of a carboxylic acid. Carboxylate salts have the general formula M(RCOO)n, where M is a metal and n is 1, 2,...; carboxylate esters have the general formula RCOOR′. R and R′ are organic groups; R′ ≠ H.
A carboxylate ion is the conjugate base of a carboxylic acid, RCOO−. It is an ion with negative charge.
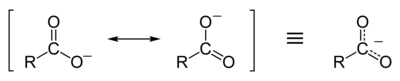
Resonance stabilization of the carboxylate ion
Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegative oxygen atoms in a resonance structure.
This delocalization of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable. In contrast, an alkoxide ion, once formed, would have a strong negative charge on the oxygen atom, which would make it difficult for the proton to escape. Carboxylic acids thus have a lower pH than alcohols: the higher the number of protons in solution, the lower the pH.[1]
Examples
- Formate ion, HCOO−
- Acetate ion, CH3COO−
- Lactate ion, CH3CH(OH)COO−
- Oxalate ion, (COO)2−
2 - Citrate ion, C
3H
5O(COO)3−
3
References
- ^ Fox, Marye Anne; Whitesell, James K. (1997). Organic Chemistry (2nd ed.). Sudbury, MA: Jones and Bartlett Publishers. ISBN 0-7637-0178-5.