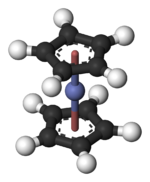
Cobaltocene
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
cobaltocene,
bis(η5- cyclopentadienyl)- cobalt | |||
| Other names
Cp2Co
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.692 | ||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [Co(η5C5H5)2] | |||
| Molar mass | 189.12 g/mol | ||
| not soluble | |||
| Structure | |||
| sandwich | |||
| zero | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
236 J.K−1.mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
+237 kJ/mol (uncertain) | ||
Std enthalpy of
combustion (ΔcH⦵298) |
-5839 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cobaltocene, known also as bis(cyclopentadienyl)cobalt(II) or even "bis Cp cobalt", is an organocobalt compound with the formula Co(C5H5)2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene. Due to the ease with which it reacts with O2, the compound must be handled and stored using air-free techniques.
Synthesis
Cobaltocene is prepared by the reaction of sodium cyclopentadienide (NaC5H5) with anhydrous cobalt(II) chloride in THF solution. Sodium chloride is cogenerated, and the organometallic product is usually purified by vacuum sublimation.[1]
Structure and bonding
In Co(C5H5)2 the Co centre is "sandwiched" between two cyclopentadienyl (Cp) rings. The Co-C bond lengths are about 2.1 Å, slightly longer than the Fe-C bond in ferrocene.[2]
Co(C5H5)2 belongs to a group of organometallic compounds called metallocenes or sandwich compounds.[3]Cobaltocene has 19 valence electrons, one more than usually found in organotransition metal complexes, such as its very stable relative ferrocene. (See 18-electron rule.) This additional electron occupies an orbital that is antibonding with respect to the Co-C bonds. Consequently, the Co-C distances are slightly longer than the Fe-C bonds in ferrocene. Many chemical reactions of Co(C5H5)2 are characterized by its tendency lose this "extra" electron, yielding 18-electron cation known as cobaltocenium:
2Co(C5H5)2 + I2 → 2[Co(C5H5)2]+ + 2I− 19e− 18e−
The otherwise close relative of cobaltocene, rhodocene does not exist as a monomer, but spontaneously dimerizes by formation of a C-C bond between Cp rings.
Reactions
Redox properties
Co(C5H5)2 is a common one-electron reducing agent in the laboratory.[4] In fact, the reversibility of the Co(C5H5)2 redox couple is so well behaved that Co(C5H5)2 may be used in cyclic voltammetry as an internal standard. Its permethylated analogue decamethylcobaltocene (Co(C5Me5)2) is an especially powerful reducing agent, due to inductive donation of electron density from the 10 methyl groups, prompting the cobalt to give up its "extra" electron even more so. These two compounds are rare examples of reductants that dissolve in non-polar organic solvents. The reduction potentials of these compounds follow, using the ferrocene-ferrocenium couple as the reference:
| Half-reaction | E° (V) |
|---|---|
| Fe(C5H5)2+ + e- ⇌ Fe(C5H5)2 | 0 (by definition) |
| Fe(C5Me5)2+ + e- ⇌ Fe(C5Me5)2 | −0.59 |
| Co(C5H5)2+ + e- ⇌ Co(C5H5)2 | −1.33 |
| Co(C5Me5)2+ e- ⇌ Co(C5Me5)2 | −1.94 |
The data show that the decamethyl compounds are ca. 600 mV more reducing than the parent metallocenes. This substituent effect is, however, overshadowed by the influence of the metal: changing from Fe to Co renders the reduction more favorable by over 1.3 volts.
Carbonylation
Treatment of Co(C5H5)2 with carbon monoxide gives the cobalt(I) derivative Co(C5H5)(CO)2, concomitant with loss of one Cp ligand.[1]
See also
References
- ^ a b King, R. B. “Organometallic Syntheses” Volume 1: Academic Press: New York, 1965.
- ^ M. Yu. Antipin, R. Boese, N. Augart, G. Schmid "Redetermination of the cobaltocene crystal structure at 100 K and 297 K: Comparison with ferrocene and nickelocene" Structural Chemistry 1993, Volume 4, Number 2, 91-101. doi:10.1007/BF00677370
- ^ C. Elschenbroich, A. Salzer ”Organometallics : A Concise Introduction” (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.