Conduritol

| |
| Names | |
|---|---|
| IUPAC name
Cyclohex-5-ene-1,2,3,4-tetrol[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10O4 | |
| Molar mass | 146.142 g·mol−1 |
| log P | −2.764 |
| Acidity (pKa) | 13.325 |
| Basicity (pKb) | 1.672 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Conduritol or 1,2,3,4-cyclohexenetetrol is any of the organic compounds with chemical formula C6H10O4, that can be seen as derivatives of cyclohexene with four hydroxyl groups (OH) replacing hydrogen atoms on the four carbon atoms not adjacent to the double bond. They are therefore cyclic polyols or cyclitols.[2]
The compounds in this group exhibit cis–trans isomerism, with six isomers that differ by the relative positions of the hydroxyls compared to the mean plane of the ring. In addition, some of these can exist as two distinct enantiomers.
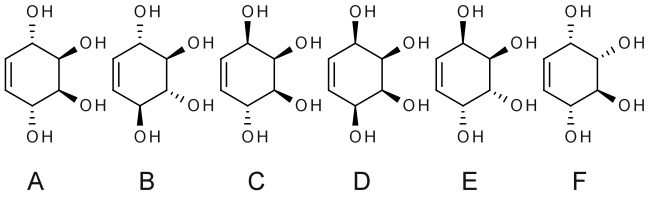
Only the A and B isomers have been found in nature. The first conduritol was isolated in 1908 by K. Kübler[3] from the bark of the vine Marsdenia condurango, hence its name. A number of conduritol derivatives has antifeedant, antibiotic, antileukemic, and growth-regulating activity. Also some conduritol derivatives show interesting biological properties including tumour-inhibitory, antileukaemic, and antibiotic activities.
See also
References
- ^ "cyclohex-5-ene-1,2,3,4-tetrol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. Retrieved 29 January 2012.
- ^ (1990) Tetrahedron, Vol 46, No 11, 3715 - 3742.
- ^ K. Kübler (1908), Arch. Phann. Ber. Stsch. Pharm. 246,620.
