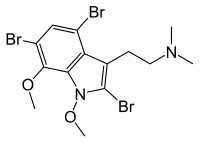
Convolutindole A
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-Tribromo-1,7-dimethoxy-N,N-dimethyltryptamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H17Br3N2O2 | |
| Melting point | 61.5-62.5 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Convolutindole A (2,4,6-tribromo-1,7-dimethoxy-N,N-dimethyltryptamine) is a brominated indole alkaloid that was first identified in 2001 in Amathia convoluta, a marine bryozoan. Bryozoans are aquatic invertebrates that grow in colonies and superficially resemble corals.
Chemistry
Convolutamine A is the 2,4,6-tribromo-1,7-dimethoxy analog of DMT, a hallucinogen that occurs naturally in many plants and animals. Other brominated derivatives of DMT include 5-bromo-DMT and 5,6-dibromo-DMT, both of which also occur in marine invertebrates.
The researchers who discovered the chemical drew specific attention to the methoxy group at the indole 1-position (attached to the nitrogen atom in the pentagonal ring) as being an unknown occurrence in the marine world until recently. 1-methoxyindoles also occur in the Brassicaceae, the plant family that cabbage and mustard belong to.
Biological activity
The chemical was tested for its ability to kill nematodes, a type of parasitic worm. It was found to be more effective in this regard than levamisole, a commercial drug used to kill parasitic worms (and in the treatment of colon cancer).
References
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (November 2013) |
- Narkowicz, C. K.; Blackman, A. J., (June 2001). Abstracts of Papers; 10th International Symposium on Marine Natural Products: Nago, Okinawa, Abstract OR1.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/np010574x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/np010574xinstead.
