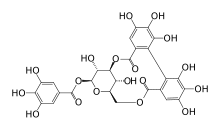
Corilagin
Appearance
| |
| Names | |
|---|---|
| IUPAC name
[3,5-dihydroxy-2-(3,4,5-trihydroxybenzoyl)oxy-6-[(3,4,5-trihydroxybenzoyl)oxymethyl]oxan-4-yl] 3,4,5-trihydroxybenzoate
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H22O18 | |
| Molar mass | 634.45 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Corilagin is an ellagitannin. Corilagin was first isolated in 1951 from Dividivi extract and from Caesalpinia coriaria,[1][2] hence the name of the molecule. It can also be found in Alchornea glandulosa and in the leaves of Punica granatum (pomegranate).[3]
It is a weak carbonic anhydrase inhibitor.[4]
References
- ^ Schmidt, O. T. .; Lademann, R. (1951). "Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe". Justus Liebigs Annalen der Chemie. 571 (3): 232. doi:10.1002/jlac.19515710305.
- ^ Schmidt, O. T.; Schmidt, D. M. (1952). "Die Umwandlung von Chebulagsäure in Corilagin XIV. Mitteilung über natürliche Gerbstoffe". Justus Liebigs Annalen der Chemie. 578: 25. doi:10.1002/jlac.19525780105.
- ^ Tanaka, T.; Nonaka, G. I.; Nishioka, I. (1985). "Punicafolin, an ellagitannin from the leaves of Punica granatum". Phytochemistry. 24 (9): 2075. doi:10.1016/S0031-9422(00)83125-8.
- ^ Satomi, H.; Umemura, K.; Ueno, A.; Hatano, T.; Okuda, T.; Noro, T. (1993). "Carbonic anhydrase inhibitors from the pericarps of Punica granatum L". Biological & Pharmaceutical Bulletin. 16 (8): 787–790. doi:10.1248/bpb.16.787. PMID 8220326.
