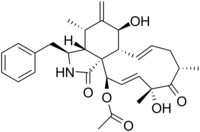
Cytochalasin D
Appearance
| |
| Names | |
|---|---|
| Other names
Zygosporin A; Cytohalasin D; Lygosporin A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.716 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H37NO6 | |
| Molar mass | 507.627 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cytochalasin D is a member of the class of mycotoxins known as cytochalasins. Cytochalasin D is an alkaloid produced by Helminthosporium and other molds.
Cytochalasin D is a cell-permeable and potent inhibitor of actin polymerization. It disrupts actin microfilaments and activates the p53-dependent pathways causing arrest of the cell cycle at the G1-S transition. It is believed to bind to F-actin polymer and prevent polymerization of actin monomers. [1]
References
- ^ Heptinstall, J. A. May H. Ratan J. R. Glenn W. L (1998). "GPIIb-IIIa antagonists cause rapid disaggregation of platelets pre-treated with cytochalasin D. Evidence that the stability of platelet aggregates depends on normal cytoskeletal assembly". Platelets. 9 (3): 227–32. doi:10.1080/09537109876744. PMID 16793707.
External links
- Cytochalasin+D at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
