Dehydroretinal
Appearance
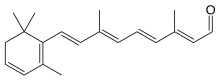
| |

| |
| Names | |
|---|---|
| Other names
3,4–didehydroretinal acetate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.781 |
| MeSH | Dehydroretinal |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H26O | |
| Molar mass | 282.42 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dehydroretinal is a derivative (metabolite) of retinal.[1] Known as vitamin A2, it is found in fish liver oils. The liver of freshwater fishes, and some fish found in India, contain a higher ratio of dehydroretinal to retinal than do other breeds.[2]
See also
References
- ^ a b Gibney, Michael J.; Margetts, Barrie M.; Kearney, John M.; et al., eds. (2012), Public Health Nutrition, John Wiley & Sons, p. 210, ISBN 1118574222.
- ^ Food and Agriculture Organization of the United Nations (1967), Requirements of Vitamin A, Thiamine, Riboflavin & Niacin: Report of a Joint Fao-Who Expert Group, United Nations, p. 26, ISBN 9251004536.
