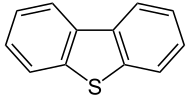
Dibenzothiophene
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
Dibenzothiophene
| |
| Other names
Diphenylene sulfide, DBT
| |
| Identifiers | |
| ECHA InfoCard | 100.004.613 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C12H8S | |
| Molar mass | 184.26 g/mol |
| Appearance | Colourless crystals |
| Density | 1.252 g/cm3 |
| Melting point | 97-100 °C(lit.) |
| Boiling point | 332-333 °C |
| insol. | |
| Solubility in other solvents | benzene and related |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
flammable |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dibenzothiophene is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It is a colourless solid that is chemically somewhat similar to anthracene. This tricyclic heterocycle, and especially its alkyl substituted derivatives, occur widely in heavier fractions of petroleum.[1]
Synthesis and reactions
Dibenzothiophene is prepared by the reaction of biphenyl with sulfur dichloride in the presence of aluminium trichloride.[2]
Reduction with lithium results in scission of one C-S bond. S-oxidation occurs to give the sulfone, which is more labile than the parent dibenzothiophene. With butyl lithium, this heterocycle undergoes stepwise lithiation at the 4- and 6- positions.
References
- ^ Teh C. Ho "Deep HDS of diesel fuel: chemistry and catalysis" Catalysis Today 2004, Volume 98, pp. 3-18. doi:10.1016/j.cattod.2004.07.048
- ^ L. H. Klemm, Joseph J. Karchesy "Dibenzothiophene from biphenyl and derivatives" Journal of Heterocyclic Chemistry, 1978, Volume 15 Issue 4, Pages 561 - 563. doi:10.1002/jhet.5570150407
