Diffusion: Difference between revisions
m Reverted edits by 168.11.166.32 (talk) to last revision by 69.77.101.174 (Report Mistake) (HG) |
|||
| Line 36: | Line 36: | ||
* [[Surface diffusion]], diffusion of adparticles on a surface |
* [[Surface diffusion]], diffusion of adparticles on a surface |
||
== fhfgh == |
|||
== See also == |
== See also == |
||
* [[advection]] |
* [[advection]] |
||
Revision as of 13:22, 5 March 2010
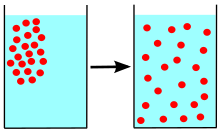
Diffusion is a time-dependent process, constituted by random motion of given entities and causing the statistical distribution of these entities to spread in space. The concept of diffusion is tied to notion of mass transfer, driven by a concentration gradient.
The concept of diffusion emerged the physical sciences. The paradigmatic examples were heat diffusion, molecular diffusion and Brownian motion. Their mathematical description was elaborated by Joseph Fourier in 1822, Adolf Fick in 1855 and by Albert Einstein in 1905.
Applications outside physics were pioneered by Louis Bachelier who in 1900 used a random walk model to describe price fluctuations on financial markets. In a less quantitative way, the concept of diffusion is invoked in the social sciences to describe the spread of ideas (Diffusion of innovations, Lexical diffusion, Trans-cultural diffusion).
Diffusion in physics
In molecular diffusion, the moving entities are small molecules. They move at random because they frequently collide. Diffusion is the resulting net transport of molecules from a region of higher concentration to one of lower concentration. Brownian motion is observed in molecules that are so large that they are not driven by their own thermal energy but by collisions with solvent particles.

While Brownian motion of large molecules is observable under a microscope, small-molecule diffusion can only be probed in carefully controlled experimental conditions. Under normal conditions, molecular diffusion is relevant only on length scales between nanometer and millimeter. On larger length scales, transport in liquids and gases is normally due to another transport phenomenon, convection.
In contrast, heat conduction through solid media is an everyday occurrence (e.g. a metal spoon partly immersed in a hot liquid). This explains why the mathematics of diffusion has first been discovered for the transport of heat, not of mass.
Other types of diffusion
- Atomic dusion, in solids.
- Eddy diffusion, incoarse-grained description of turbulent flow
- Effusion of a gas through small holes.
- Electronic diffusion, resulting in an electric current called the diffusion current.
- Facilitated diffusion, present in some organisms.
- Gaseous diffusion, used for isotope separation
- Heat equation, diffusion of thermal energy
- Itō diffusion, mathematisation of Brownian motion, continuous stochastic process.
- Knudsen diffusion of gas in long pores with frequent wall collisions
- Momentum diffusion, ex. the diffusion of the hydrodynamic velocity field
- Osmosis is the diffusion of water through a cell membrane.
- Photon diffusion
- Reverse diffusion, against the concentration gradient, in phase separation
- Rotational diffusion, random reorientions of molecules
- Surface diffusion, diffusion of adparticles on a surface
