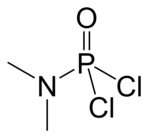
Dimethylamidophosphoric dichloride

| |
| |
| Names | |
|---|---|
| IUPAC name
N-Dichlorophosphoryl-N-methylmethanamine
| |
| Other names
(Dimethylamido)phosphoryl dichloride
N,N-Dimethylphosphoramidodichloridate | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6Cl2NOP | |
| Molar mass | 161.95 g·mol−1 |
| Melting point | < 0 °C (32 °F; 273 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethylamidophosphoric dichloride is an important chemical for few industrial purposes. It is an important chemical for synthesizing phosphoramidates as well as Nerve agent GA which is used as a chemical weapon.
Safety
This chemical is also corrosive, flammable and will cause mild nerve agent symptoms if ingested or absorbed through skin due to its nature. It will react with water giving off hydrogen chloride vapors and dimethylamidophosphoric acid.

