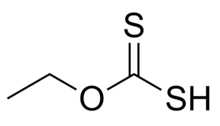
Ethyl xanthic acid
| |
| Names | |
|---|---|
| IUPAC name
Ethoxymethanedithioic acid[1]
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| CH3CH2OCS2H | |
| Molar mass | 122.20 g·mol−1 |
| Appearance | Colorless oily liquid[2][1] |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | Decomposes |
| Slightly[1] | |
| Acidity (pKa) | 1.6[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl xanthic acid is an organic compound with the chemical formula CH3CH2−O−C(=S)−SH. It can be viewed as an O-ethyl ester of dithiocarbonic O,S-acid (the formula of that acid is S=C(OH)(SH)). Ethyl xanthic acid belongs to the category of thioacids, where the prefix thio- means that an oxygen atom in the compound is replaced by a sulfur atom.
Preparation
[edit]Ethyl xanthic acid is obtained by the action of dilute sulfuric acid on potassium ethyl xanthate at 0 °C.[4]
Ethyl xanthic acid is a colorless, labile oil. In aqueous solution, it decomposes rapidly by a unimolecular pathway to give carbon disulfide and ethanol.[1][4][5]
Esters of ethyl xanthic acid
[edit]The methyl and ethyl esters of ethyl xanthic acid are colorless, oily liquids with a penetrating odor.[6]
Reactions
[edit]Ethyl xanthic acid reacts with water or moisture producing carbon disulfide.[1][clarification needed]
Safety
[edit]In an experiment with white rats, chronically exposed rats by inhalation of ethyl xanthic acid revealed higher frequency of chromosomal rearrangements in lymphocytes of peripheral blood than the control rats.[1]
References
[edit]- ^ a b c d e f g h i j k l m n o p "Ethylxanthate". PubChem.
- ^ "Xanthic acid". merriam-webster.com.
- ^ Millican, Robert J.; Sauers, Carol K. (1979). "General acid-catalyzed decomposition of alkyl xanthates". The Journal of Organic Chemistry. 44 (10): 1664–1669. doi:10.1021/jo01324a018.
- ^ a b . Encyclopædia Britannica. Vol. 28 (11th ed.). 1911. p. 881.
- ^ Iwasaki, Iwao; Cooke, Strathmore R. B. (1958). "The Decomposition of Xanthate in Acid Solution". Journal of the American Chemical Society. 80 (2): 285–288. doi:10.1021/ja01535a008.
- ^ "Xanthic acid". dictionary.com.


