Fluoroantimonic acid

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Fluoroantimonic acid | |
| Systematic IUPAC name
Hydrogen hexafluoro-λ5-stibanuide | |
| Other names
H(+) hexafluoro-λ5-stibanuide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.279 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HSbF6 | |
| Molar mass | 236.808 g/mole |
| Appearance | colourless syrup |
| Boiling point | decomposes |
| decomposes | |
| Acidity (pKa) | −25 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
HBF4 |
Other cations
|
NaPF6, NaSbF6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
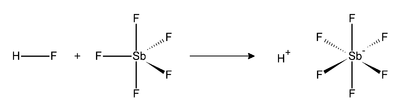
Fluoroantimonic acid (HSbF6) is a mixture of hydrogen fluoride and antimony pentafluoride in various ratios.[1] The 1:1 combination forms the strongest known superacid, which has been demonstrated to protonate even hydrocarbons to afford carbocations and H2.[2]
The reaction of hydrogen fluoride (HF) and SbF5 is exothermic. HF releases its proton (H+), and its conjugate base (F−) is sequestered by one of more molecules SbF5 to give the octahedral SbF6−. This anion is classified as noncoordinating, because it is both a very weak nucleophile and a very weak base. The proton effectively becomes "naked", which accounts for the system's extreme acidity. Fluoroantimonic acid is 2×1019 (20 quintillion) times stronger than 100% sulfuric acid.[3]
Structure
Two related products have been crystallised from HF-SbF5 mixtures, and both have been analyzed by single crystal X-ray crystallography. These salts have the formulas [H2F+][Sb2F11−] and [H3F2+][Sb2F11−]. In both salts the anion is Sb2F11−.[4] As mentioned above, SbF6− is classified as weakly basic; the larger monoanion Sb2F11− would be expected to be still weaker.
Comparison with other acids
The following values[citation needed] are based upon the Hammett acidity function. Acidity is indicated by large negative values of H0.
- Fluoroantimonic acid (1990) (H0 Value = −31.3)
- Magic acid (1974) (H0 Value = −19.2)
- Carborane superacid (1969) (H0 Value = −18.0)
- Fluorosulfuric acid (1944) (H0 Value = −15.1)
- Triflic acid (1940) (H0 Value = −14.9)
Applications
This extraordinarily strong acid protonates nearly all organic compounds. In 1967, Bickel and Hogeveen showed that HF-SbF5 will remove H2 from isobutane and methane from neopentane:[5][6]
- (CH3)3CH + H+ → (CH3)3C+ + H2
- (CH3)4C + H+ → (CH3)3C+ + CH4
Safety
HF-SbF5 is rapidly and explosively decomposed by water. It reacts with virtually all known solvents.[1] Solvents that have been proven to be compatible with HF-SbF5 are SO2ClF and liquefied sulfur dioxide. Chlorofluorocarbons have also been used as solvents. Containers for HF-SbF5 are made of PTFE.
See also
References
- ^ a b Olah, G. A.; Prakash, G. K. S.; Wang, Q.; Li, X. “Hydrogen Fluoride–Antimony(V) Fluoride” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
- ^ George Andrew Olah (2001). A life of magic chemistry: autobiographical reflections of a nobel prize winner. John Wiley and Sons. pp. 100–101. ISBN 0471157430.
- ^ Olah, George A. (2005). "Crossing Conventional Boundaries in Half a Century of Research". J. Org. Chem. 70 (7): 2413–2429. doi:10.1021/jo040285o. PMID 15787527.
- ^ Mootz, D.; Bartmann, K. (1988). "The Fluoronium Ions H2F+ and H3F2+: Characterization by Crystal Structure Analysis". Angewandte Chemie, International Edition in English. 27: 391–392. doi:10.1002/anie.198803911.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bickel, A. F.; Gaasbeek, C. J.; Hogeveen, H.; Oelderik, J. M.; Platteeuw, J. C. (1967). "Chemistry and spectroscopy in strongly acidic solutions: reversible reaction between aliphatic carbonium ions and hydrogen". Chemical Communications. 1967: 634–5. doi:10.1039/C19670000634.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hogeveen, H.; Bickel, A. F. (1967). "Chemistry and spectroscopy in strongly acidic solutions: electrophilic substitution at alkane-carbon by protons". Chemical Communications. 1967: 635–6. doi:10.1039/C19670000635.
{{cite journal}}: CS1 maint: multiple names: authors list (link)