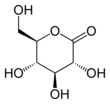
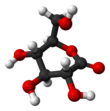
Glucono-δ-lactone
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-one
| |||
| Other names
D-Glucono-1,5-lactone
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.833 | ||
| EC Number |
| ||
| E number | E575 (acidity regulators, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H10O6 | |||
| Molar mass | 178.140 g·mol−1 | ||
| Melting point | 150–153 °C (302–307 °F; 423–426 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Glucono delta-lactone (GDL), also known as gluconolactone, is a food additive with the E number E575[3] used as a sequestrant, an acidifier,[4] or a curing, pickling, or leavening agent. It is a lactone of D-gluconic acid. Pure GDL is a white odorless crystalline powder.
GDL is commonly found in honey, fruit juices, personal lubricants, wine [citation needed], and has been marketed for use in feta cheese.[5] GDL is neutral, but hydrolyses in water to gluconic acid which is acidic, adding a tangy taste to foods, though it has roughly a third of the sourness of citric acid. It is metabolized to glucose; one gram of GDL yields roughly the same amount of metabolic energy as one gram of sugar.
Upon addition to water, GDL is partially hydrolysed to gluconic acid, with the balance between the lactone form and the acid form established as a chemical equilibrium. The rate of hydrolysis of GDL is increased by heat and high pH.[6]
The yeast Saccharomyces bulderi can be used to ferment gluconolactone to ethanol and carbon dioxide. The pH value greatly affects culture growth. Gluconolactone at 1 or 2% in a mineral media solution causes the pH to drop below 3.[7]
See also
References
- ^ Budavari, Susan, ed. (2001), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.), Merck, ISBN 0911910131, 4469.
- ^ Beil. 18, V, 5, 11
- ^ Current EU approved additives and their E Numbers, Food Standards Agency
- ^ Martin, F.; Cayot, N.; Marin, A.; et al. (2009). "Effect of oxidoreduction potential and of gas bubbling on rheological properties and microstructure of acid skim milk gels acidified with glucono-δ-lactone". Journal of Dairy Science. 92 (12): 5898–5906. doi:10.3168/jds.2009-2491. PMID 19923593.
- ^ Blythman, Joanna (21 February 2015). "Inside the food industry: the surprising truth about what you eat". The Guardian. Retrieved 28 October 2016.
- ^ Pocker, Y.; Green, Edmond (1973). "Hydrolysis of D-Glucono-δ-lactone. I. General Acid–Base Catalysis, Solvent Deuterium Isotope Effects, and Transition State Characterization". J. Am. Chem. Soc. 95 (1): 113–19. doi:10.1021/ja00782a019. PMID 4682891.
- ^ Van Dijken, J. P.; Van Tuijl, A.; Luttik, M. A.; et al. (2002). "Novel pathway for alcoholic fermentation of delta-gluconolactone in the yeast Saccharomyces bulderi". Journal of Bacteriology. 184 (3): 672–678. doi:10.1128/JB.184.3.672-678.2002. PMC 139522. PMID 11790736.