Guerbet reaction
The Guerbet reaction, named after Marcel Guerbet (1861–1938), is an organic reaction converting a primary aliphatic alcohol into its β-alkylated dimer alcohol with loss of one equivalent of water.[1] This reaction requires a catalyst and elevated temperatures.

The original 1899 publication concerned the conversion of n-butanol to 2-ethylhexanol. The alcohols derived from this reaction are called Guerbet alcohols. Application of long-chained aliphatic alcohols gives access to surfactants.
The reaction requires alkali metal hydroxides or alkoxides and hydrogenation catalysts such as Raney Nickel at higher temperature (220 °C) and pressure.
Reaction mechanism
The reaction mechanism for this reaction is a four-step sequence. In the first step the alcohol is oxidized to the aldehyde. These intermediates then react in an aldol condensation to the vinyl aldehyde which the hydrogenation catalyst then reduces to the alcohol.[2]

The Cannizzaro reaction is a competing reaction when two aldehyde molecules react by disproportionation to form the corresponding alcohol and carboxylic acid. Another side reaction is the Tishchenko reaction.
Scope
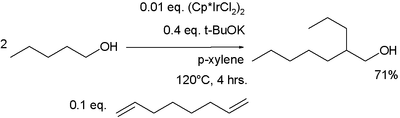
New catalyst systems are actively researched which can bring down the processing temperature. In one study 1-pentanol is reacted with an iridium dehydrogenation catalyst (Cp* stands for the pentamethylcyclopentadiene ligand) and potassium tert-butoxide as a base in p-xylene.[3] A small amount of the diene 1,7-octadiene is required as a proton acceptor.
See also
- Guerbet alcohols
References
- ^ Marcel Guerbet (1909). "Condensation de l'alcool isopropylique avec son dérivé sodé; formation du méthylisobutylcarbinol et du diméthyl-2.4-heptanol-6". Comptes rendus de l'Académie des sciences. 149: 129–132.
- ^ S. Veibel; J. I. Nielsen (1967). "On the mechanism of the Guerbet reaction". Tetrahedron. 23 (4): 1723–1733. doi:10.1016/S0040-4020(01)82571-0.
- ^ Toyomi Matsu-ura; Satoshi Sakaguchi; Yasushi Obora; Yasutaka Ishii (2006). "Guerbet Reaction of Primary Alcohols Leading to -Alkylated Dimer Alcohols Catalyzed by Iridium Complexes". Journal of Organic Chemistry. 71 (21): 8306–8308. doi:10.1021/jo061400t. PMID 17025333.
External links
- A Review of Guerbet Chemistry Anthony J. O’Lenick, Jr. http://www.zenitech.com Link
