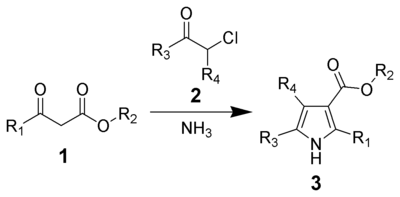
Hantzsch pyrrole synthesis
The Hantzsch Pyrrole Synthesis, named for Arthur Rudolf Hantzsch, is the chemical reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).[1][2] Pyrroles are found in a variety of natural products with biological activity, so the synthesis of substituted pyrroles has important applications in medicinal chemistry.[3] Alternative methods for synthesizing pyrroles exist, such as the Knorr Pyrrole Synthesis and Paal-Knorr Synthesis.
Mechanism
Below is one published mechanism for the reaction:[4]

The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an enamine (3). The enamine then attacks the carbonyl carbon of the α-haloketone (4). This is followed by the loss of H2O, giving an imine (5). This intermediate undergoes an intramolecular nucleophilic attack, forming a 5-membered ring (6). Finally, a hydrogen is eliminated and the pi-bonds are rearranged in the ring, yielding the final product (7).
An alternative mechanism has been proposed in which the enamine (3) attacks the α-carbon of the α-haloketone (4) as part of a nucleophilic substitution, instead of attacking the carbonyl carbon.[5]
Generalized Reaction
A generalization of the Hantzsch Pyrrole Synthesis was developed by Estevez, et al.[6] In this reaction highly substituted pyrroles can be synthesized in a one-pot reaction, with relatively high yields (60% - 97%). This reaction involves the high-speed vibration milling (HSVM) of ketones with N-iodosuccinimide (NIS) and p-toluenesulfonic acid, to form an α-iodoketone in situ. This is followed by addition of a primary amine, a β-dicarbonyl compound, cerium(IV) ammonium nitrate (CAN) and silver nitrate, as shown in the scheme below:

Applications
2,3-dicarbonylated pyrroles
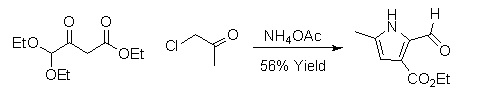
2,3-dicarbonylated pyrroles can be synthesized by a version of the Hantzsch Pyrrole Synthesis.[7] These pyrroles are particularly useful for total synthesis because the carbonyl groups can be converted into a variety of other functional groups.
Substituted Indoles
The reaction can also occur between an enamine and an α-haloketone to synthesize substituted indoles, which also have biological significance.[5][8]

Continuous Flow Chemistry
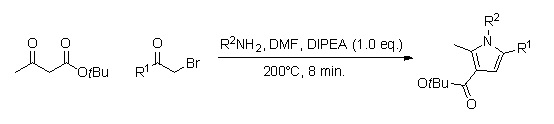
A library of substituted pyrrole analogs can be quickly produced by using continuous flow chemistry (reaction times of around 8 min.).[9] The advantage of using this method, as opposed to the in-flask synthesis, is that this one does not require the work-up and purification of several intermediates, and could therefore lead to a higher percent yield.
See Also
References
- ^ Hantzsch, A. Ber. 1890, 23, 1474.
- ^ Feist, F. Ber. 1902, 35, 1538.
- ^ Furstner, A. Angew. Chem. Int. Ed. 2003, 42¸ 3582-3603.
- ^ Li, J.J. Name Reactions; 4th ed.; Springer-Verlag: Berlin, Germany, 2009; p. 276.
- ^ a b Wang, Zerong. Comprehensive Organic Name Reactions and Reagents, 3 Volume Set; John Wiley & Sons, Hoboken, New Jersey, 2009; pp. 1326-1327.
- ^ Estevez, V.; Villacampa, M.; Menendez, J.C. Chem. Commun. 2012, 49, 591-593.
- ^ Moss, T.A.; Nowak, T. Tetrahedron Lett. 2012, 53, 3056-3060.
- ^ Jones, C.D; Suarez, T. J. Org. Chem. 1979, 37, 3622-3623.
- ^ Herath, A.; Cosford, N.D.P. Org. Lett. 2010, 12, 5182-5185.
