Hemiaminal
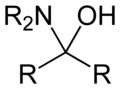
A hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: -C(OH)(NR2)-. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution.[1]
Examples
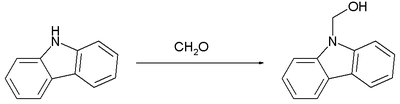
A hemiaminal is the first step in the reaction of an aldehyde or ketone with an amine. Being one of the most reactive carbonyls, formaldehyde is well known to give carbinolamines. Illustrative is the reaction of the weakly basic secondary amine carbazole with formaldehyde.[2]
As is typical with a secondary amine derivative, this carbinol converts readily to the methylene-linked bis(carbazole).
Those generated from primary amines are unstable to the extent that they have never been isolated and very rarely been observed directly. In a 2007 study a hemiaminal substructure trapped in the cavity of a host-guest complex was studied with a chemical half-life of 30 minutes. Because both amine and carbonyl group are isolated in a cavity, hemiaminal formation is favored due to a high forward reaction rate comparable to an intramolecular reaction and also due to restricted access of external base (another amine) to the same cavity which would favor elimination of water to the imine.[3]
Hemiaminal formation is a key step in an asymmetric total synthesis of saxitoxin:[4]
In this reaction step the alkene group is first oxidized to an intermediate acyloin by action of osmium(III) chloride, oxone (sacrificial catalyst) and sodium carbonate (base).
Ammonia-aldehydes
The adducts formed by the addition of ammonia to aldehydes have long been studied.[5] This class of compounds contain both a primary amino group and a hydroxyl group bonded to the same carbon atom. These species have rarely been detected, much less isolated in bulk. They are invoked as intermediates in the formation of Schiff bases and related imines from the reaction of ammonia and aldehydes and ketones.
| Chemical Formula | Molecular Weight (g/mol) |
Chiral? | Image | Image2 | Name |
|---|---|---|---|---|---|
| CH5NO | 47.06 | No |  |
N/A | Methanolamine / Aminomethanol |
| C2H7NO | 61.08 | Yes |  |
 |
1-Aminoethanol |
| C2H7NO2 | 77.08 | Yes |  |
 |
Aminoethylene glycol |
| C2H7NOS | 93.15 | Yes |  |
 |
Aminoethane-2-thiol |
| C3H9NO | 75.08 | Yes |  |
 |
1-Aminopropanol |
| C3H9NO | 75.08 | No |  |
N/A | 2-Amino-2-propanol |
| C3H9NO2 | 91.15 | Yes |  |
 |
1-Amino-1,2-propanediol |
| C4H11NO | 89.14 | Yes |  |
 |
2-Methyl-1-amino-1-propanol |
| C4H11NO | 89.14 | Yes |  |
 |
1-Aminobutan-1-ol |
| C4H11NO | 89.14 | Yes |  |
 |
2-Aminobutan-2-ol |
| C4H11NOS | 121.2 | Yes |  |
 |
1-Amino-3-methylmercapto-1-propanol |
| C3H9NOS | 107.17 | Yes |  |
 |
1-Amino-2-methylmercapto-1-ethanol |
| C4H9NO | 87.12 | Yes |  |
 |
2-Hydroxypyrrolidine |
| C5H13NO | 103.16 | Yes |  |
 |
1-Aminopentan-1-ol |
| C5H13NO | 103.16 | Yes |  |
 |
1-Amino-3-methyl-1-butanol |
| C5H13NO | 103.16 | Yes |  |
 |
1-Amino-2-methyl-1-butanol |
| C5H13NO | 103.16 | Yes |  |
 |
2-Aminopentan-2-ol |
| C5H13NO | 103.16 | No |  |
N/A | 3-Aminopentan-3-ol |
| C6H13NO | 115.16 | No |  |
N/A | 1-Aminocyclohexan-1-ol |
| C8H11NO | 137.18 | Yes |  |
 |
Toluylmethanolamine |
| C8H11NO2 | 153.18 | Yes |  |
 |
para-Hydroxytoluylmethanolamine |
| C10H12N2O | 176.22 | Yes |  |
 |
1-Amino-2-(1H-indol-3-yl)-1-ethanol |
See also
References
- ^ Urbansky, Edward T. "Carbinolamines and geminal diols in aqueous environmental organic chemistry Journal of Chemical Education 2000, volume 77, 1644-1647. doi:10.1021/ed077p1644
- ^ Carbazol-9-yl-methanol Milata Viktora, Kada Rudolfa, Lokaj J¨¢nb Molbank 2004, M354 open access publication [1]
- ^ Stabilization of Labile Carbonyl Addition Intermediates by a Synthetic Receptor Tetsuo Iwasawa, Richard J. Hooley, Julius Rebek Jr. Science 317, 493 (2007) doi:10.1126/science.1143272
- ^ (+)-Saxitoxin: A First and Second Generation Stereoselective Synthesis James J. Fleming, Matthew D. McReynolds, and J. Du Bois J. Am. Chem. Soc., 129 (32), 9964 -9975, 2007. doi:10.1021/ja071501o
- ^ Justus Liebig "Ueber die Producte der Oxydation des Alkohols" Annalen der Pharmacie 1835, Volume 14, pp 133–167. doi:10.1002/jlac.18350140202