Hofmann rearrangement
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.[1][2][3]
The reaction is named after its discoverer: August Wilhelm von Hofmann. This reaction is also sometimes called the Hofmann degradation, and should not be confused with the Hofmann elimination.
Mechanism
The reaction of bromine with sodium hydroxide forms sodium hypobromite in situ, which transforms the primary amide into an intermediate isocyanate. The intermediate isocyanate is hydrolyzed to a primary amine giving off carbon dioxide.
Variations
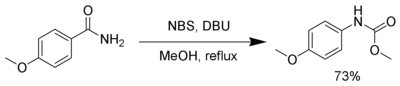
Several reagents can substitute for bromine. N-Bromosuccinimide and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) can effect a Hofmann rearrangement. In the following example, the intermediate isocyanate is trapped by methanol forming a carbamate.[4]
In a similar fashion the intermediate isocyanate can be trapped by tert-butanol yielding the t-butoxycarbonyl (Boc)-protected amine.
A mild alternative to bromine is also (bis(trifluoroacetoxy)iodo)benzene.[5]
Applications
- Aliphatic & Aromatic amides are converted into aliphatic & aromatic amines respectively.
- In the preparations of Anthranilic Acid from Phthalimide.
- Nicotinic acid is converted into 3-Amino pyridine.
- The Symmetrical structure of α-phenyl propanamide does not change after hofmann reaction.
See also
- Beckmann rearrangement
- Curtius rearrangement
- Iodoform reaction
- Lossen rearrangement
- Schmidt reaction
- Weerman degradation
References
- ^ Hofmann, A. W. v. Ber. 1881, 14, 2725.
- ^ Wallis, E. S.; Lane, J. F. Org. React. 1949, 3, 267-306. (Review)
- ^ Shioiri, T. Comp. Org. Syn. 1991, 6, 800-806. (Review)
- ^ Keillor, J. W.; Huang, X. - Organic Syntheses (2004, 549pp)
- ^ Almond, M. R.; Stimmel, J. B.; Thompson, E. A.; Loudon, G.M. - Organic Syntheses (1993, 132pp)
External links
Bibliography
- Clayden, Jonnathan (2007). Organic Chemistry. Oxfort University Press Inc. p. 1073. ISBN 978-0-19-850346-0.
- Fieser, Louis F. (1962). Advanced Organic Chemistry. Reinhold Publishing Corporation, Chapman & Hall, Ltd. pp. 499–501.