Iminium
An iminium cation in organic chemistry is a functional group with the general structure [R1R2C=NR3R4]+.[1] They are common in synthetic chemistry and biology.
Structure
Imininium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed.

Formation
Iminium cations are obtained by protonation and alkylation of imines:
- RN=CR'2 + H+ → [RNH=CR'2]+
- RN=CR'2 + R"+ → [RR"N=CR'2]+
They also are generated by the condensation of secondary amines with ketones or aldehydes:
- O=CR'2 + R2NH + H+ [R2N=CR'2]+ + H2O
This rapid, reversible reaction is one step in "iminium catalysis".[3]
More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine.[4]
Occurrence
Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are encountered in synthetic organic chemistry.
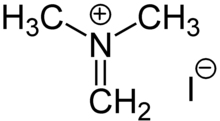
Reactions
Iminium salts hydrolyse to give the corresponding ketone or aldehyde:[6]
- [RR"N=CR'2]+ + H2O → [RR"NH2]+ + O=CR'2
Iminium cations are readily reduced to the amines, e.g. by sodium cyanoborohydride. They are intermediates in the reductive amination of ketones and aldehydes.
Named reactions involving iminium species
- Aza-Cope rearrangement[7]
- Beckmann rearrangement
- Duff reaction
- Mannich reaction
- Pictet-Spengler reaction
- Stephen reaction
- Stork enamine alkylation
- Vilsmeier-Haack reaction and Vilsmeier reagent
Iminylium ions
Iminylium ions have the general structure R2C=N+. They form a subclass of nitrenium ions.[8]
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "iminium compounds". doi:10.1351/goldbook.I02958
- ^ "Electrophilic Reactivity of a 2‐Azaallenium and of a 2‐Azaallylium Ion". European Journal of Organic Chemistry. 2000: 1589–1593. doi:10.1002/(SICI)1099-0690(200004)2000:8<1589::AID-EJOC1589>3.0.CO;2-T.
{{cite journal}}: Unknown parameter|authors=ignored (help). - ^ Erkkilä, Anniinä; Majander, Inkeri; Pihko, Petri M. (2007). "Iminium Catalysis". Chem. Rev. 107: 5416–5470. doi:10.1021/cr068388p.
- ^ Hafner, = Klaus; Meinhardt, Klaus-Peter (1984). "Azulene". 62: 134. doi:10.15227/orgsyn.062.0134.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: extra punctuation (link) - ^ E. F. Kleinman (2004). "Dimethylmethyleneammonium Iodide and Chloride". Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rd346.
- ^ "A General Synthesis of Cyclobutanones from Olefins and Tertiary Amides: 3-Hexylcyclobutanone". Org. Synth. 69: 199. 1990. doi:10.15227/orgsyn.069.0199.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Grieco, P. A.; Larsen, S. D. (1990). "Iminium Ion-Based Diels–Alder Reactions: N-Benzyl-2-Azanorborene" (PDF). Organic Syntheses. 68: 206. doi:10.15227/orgsyn.068.0206.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "iminylium ions". doi:10.1351/goldbook.I02964

