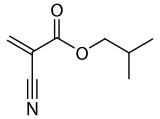
Isobutyl cyanoacrylate
Appearance
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropyl 2-cyanoprop-2-enoate | |
| Other names
2-Methylpropyl 2-cyanopropenoate
Isobutyl 2-cyanoacrylate Bucrylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.690 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H11NO2 | |
| Molar mass | 153.181 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isobutyl cyanoacrylate is an isomer of butyl cyanoacrylate. It is used in medical procedures either to close incisions and lacerations without the use of sutures, or as an adjunct to strengthen the suturing.[1] This use is possible because it is a bactericidal liquid monomer which, in the presence of small amounts of moisture, rapidly polymerizes to form a strong adhesive.[2]
References
[edit]- ^ de Blanco LP (February 1994). "Lip suture with isobutyl cyanoacrylate". Endodontics & Dental Traumatology. 10 (1): 15–8. doi:10.1111/j.1600-9657.1994.tb00592.x. PMID 8005074.
- ^ J. E. Hale (July 1970). "Isobutyl cyanoacrylate as a skin". Postgraduate Medical Journal. 46 (537): 447–450. doi:10.1136/pgmj.46.537.447. PMC 2467054. PMID 5476147.
