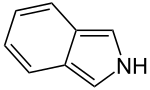
Isoindole
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
2H-Isoindole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H7N | |
| Molar mass | 117.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isoindole in heterocyclic chemistry is a benzo-fused pyrrole.[1] The compound is an isomer of indole and its reduced form is an isoindoline.
In solution its tautomer without full aromaticity over the whole ring system is predominant:[citation needed]
and therefore the compound resembles a pyrrole more than a simple imine. Isoindoles are building blocks for phthalocyanines which are relevant as dyes
Isoindole-1,3-diones
The commercially important phthalimide is an isoindole-1,3-dione with two carbonyl groups attached to the heterocyclic ring. Thalidomide is an infamous drug based on this framework.
See also
- Isoindene with nitrogen replaced by a methylene group.
References
- ^ Gilchrist, T. L. (1987). Heterocyclic Chemistry. Longman. ISBN 0-582-01422-0.

