Isosbestic point
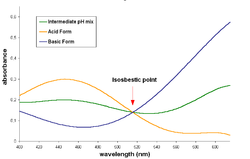
In spectroscopy, an isosbestic point is a specific wavelength at which two (or more) chemical species have the same Molar absorptivity(ε). This point represents a wavelength on an isosbestic plot at which the absorption spectra of two species cross each other, the latter statement being true only if the molar absorptivities are represented (instead of absorbances), or if both species are in the same concentration. In other terms, for two or more species, the isosbestic point is a wavelength where they have equal extinction coefficients.
A pair of substances can have several isosbestic points in their spectra.
When a 1-to-1 (one mole of reactant gives one mole of product) chemical reaction (including equilibria) involves a pair of substances with an isosbestic point in common, the absorbance of the reaction mixture at this wavelength keeps invariant, regardless of the extent of reaction (or the position of the chemical equilibrium). This occurs because the two substances absorb light of that specific wavelength to the same extent, and the analytical concentration remains constant.
For the reaction:
the analytical concentration is the same at any point in the reaction:
- .
The absorbance of the reaction mixture (assuming it depends only on X and Y) is:
- .
But at the isosbestic point both molar absorptivities are the same:
- .
Hence, the absorbance
does not depend on the extent of reaction (i.e. in the particular concentrations of X and Y)
Applications

In chemical kinetics, isosbestic points are used as reference points in the study of reaction rates, as the absorbance at those wavelengths remains constant throughout the whole reaction.
Isobestic points are used in medicine in a laboratory technique called oxymetry to determine hemoglobin concentration, regardless of its saturation. Oxyhaemoglobin and deoxyhaemoglobin have isosbestic points at 590 nm and 805 nm.
Isosbestic points are also used in clinical chemistry, as a quality assurance method, to verify the accuracy in the wavelength of a spectrophotometer. This is done by measuring the spectra of a standard substance at two different pH conditions (above and below the pKa of the susbtance). The standards used include potassium dichromate (isosbestic points at 339 and 445 nm), bromothymol blue (325 and 498 nm) and congo red (541 nm). The wavelength of the isosbestic point determined does not depend on the concentration of the substance used, and so, it becomes a very reliable reference.





