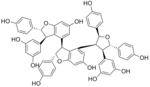
Kobophenol A
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
5-[(2S,3R,4S,5S)-4-[(2S,3S)-3-[(2R,3R)-3-(3,5-dihydroxyphenyl)-6-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-4-yl]-6-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-4-yl]-2,5-bis(4-hydroxyphenyl)oxolan-3-yl]benzene-1,3-diol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C56H44O13 | |||
| Molar mass | 924.94 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Kobophenol A is a compound with the formula C56H44O13. It can be thought of as a tetramer of the stilbene analog C14H12O4 with the loss of two water molecules and an oxygen atom (whether or not this is how it is formed). It can be isolated from Caragana chamlagu[1] and from Caragana sinica.[2]
It has been shown to inhibit acetylcholinesterase.[1]
Acid-catalyzed epimerization of kobophenol A to carasinol B can be performed in vitro.[3]
References
- ^ a b (+)-α-Viniferin, a Stilbene Trimer from Caragana chamlague, Inhibits Acetylcholinesterase. Sang Hyun Sung, So Young Kang, Ki Yong Lee, Mi Jung Park, Jeong Hun Kim, Jong Hee Park, Young Chul Kim, Jinwoong Kim and Young Choong Kim, Biological & Pharmaceutical Bulletin, Vol. 25, 2002
- ^ Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method. Shu Na; Zhou Hong; Hu Changqi, Biological & pharmaceutical bulletin, 2006, vol. 29, no4, pp. 608-612
- ^ Acid-catalyzed Epimerization of Kobophenol A to Carasinol B. Kejun Cheng, Gaolin Liang and Changqi Hu, Molecules 2008, 13(4), 938-942