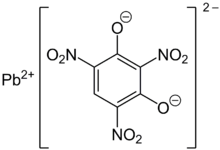
Lead styphnate
| |
| Names | |
|---|---|
| IUPAC name
Lead 2,4,6-trinitrobenzene-1,3-diolate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.703 |
PubChem CID
|
|
| UN number | 0130 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6HN3O8Pb | |
| Molar mass | 450.288 g/mol |
| Density | 3.02 g/cm3, solid |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lead styphnate (lead 2,4,6-trinitroresorcinate, C6HN3O8Pb ), whose name is derived from styphnic acid, is an explosive used as a component in primer and detonator mixtures for less sensitive secondary explosives.
There are two forms of lead styphnate: six-sided monohydrate crystals and small rectangular crystals. Lead styphnate varies in color from yellow to brown. Lead styphnate is particularly sensitive to fire and the discharge of static electricity. When dry, it can be readily detonated by static discharges from the human body. The longer and narrower the crystals, the more susceptible lead styphnate is to static electricity. Lead styphnate does not react with metals and is less sensitive to shock and friction than mercury fulminate or lead azide. Lead styphnate is only slightly soluble in water and methyl alcohol and may be neutralized by a sodium carbonate solution. It is stable in storage, even at elevated temperatures.
As with other lead-containing compounds, lead styphnate is inherently toxic to humans if ingested i.e. can cause heavy metal poisoning.
