MCM-41
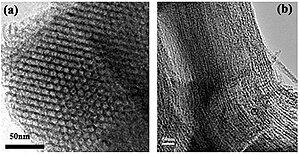
MCM-41 (Mobil Composition of Matter No. 41) is a mesoporous material with a hierarchical structure from a family of silicate and alumosilicate solids that were first developed by researchers at Mobil Oil Corporation[2] and that can be used as catalysts or catalyst supports.[3]
Structure
[edit]MCM-41 consists of a regular arrangement of cylindrical mesopores that form a one-dimensional pore system.[3] It is characterized by an independently adjustable pore diameter, a sharp pore distribution, a large surface and a large pore volume. The pores are larger than with zeolites and the pore distribution can easily be adjusted.[4] The mesopores have a diameter of 2 nm to 6.5 nm.
Properties
[edit]Contrary to zeolites, the framework of MCM-41 has no bronsted acid centers because there is no aluminium contained in the lattice. The acidity of alumina-doped MCM-41 therefore is comparable to that of the amorphous alumosilicates.[4]
MCM-41 is not hydrothermally stable because of the slight wall thickness and the low degree of cross-linking of the silicate units.[3]
Synthesis
[edit]To achieve a defined pore diameter surfactants are used that form micelles in the synthesis solution. These micelles form templates that help build up the mesoporous framework. For MCM-41 mostly cetyltrimethylammonium bromide (CTAB) is used.
The surfactant first forms rod-like micelles that subsequently align into hexagonal arrays. After adding silica species these cover the rods. Later, calcination leads to a condensation of the silanol groups so that the silicon atoms are bridged by oxygen atoms. The organic template is oxidized and disappears.
Uses
[edit]MCM-41, as the zeolites, are widely used as catalytic cracking.[5] MCM-41 type materials have been widely used as support of heterogeneous catalysts [6] and also used for separations.
References
[edit]- ^ Guo, M.; Wang, H.; Huang, D.; Han, Z.; Li, Q.; Wang, X.; Chen, J. (2014). "Amperometric catechol biosensor based on laccase immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/PVA matrix". Science and Technology of Advanced Materials. 15 (3): 035005. Bibcode:2014STAdM..15c5005G. doi:10.1088/1468-6996/15/3/035005. PMC 5090526. PMID 27877681.
- ^ Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. (1992). "Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism". Nature. 359 (6397): 710–712. doi:10.1038/359710a0. ISSN 0028-0836. S2CID 4249872.
- ^ a b c Reichinger, M. (2007) Poröse Silikate mit hierarchischer Porenstruktur: Synthese von mikro-/mesoporösem MCM-41 und MCM-48 Materialien aus zeolithischen Baueinheiten des MFI-Gerüststrukturtyps, Dissertation Ruhr-Universität Bochum (in German)
- ^ a b Silaghi, M.-C., Chizallet, C., Raybaud, P. (2014). "Challenges on molecular aspects of dealumination and desilication of zeolites". Microporous and Mesoporous Materials. 191: 82–96. doi:10.1016/j.micromeso.2014.02.040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sayari, Abdelhamid (1996). "Catalysis by Crystalline Mesoporous Molecular Sieves". Chemistry of Materials. 8 (8): 1840–1852. doi:10.1021/cm950585+.
- ^ P. Chatterjee; H. Wang; J. S. Manzano; U. Kanbur; A. D. Sadow; I. I. Slowing (2022). "Surface ligands enhance the catalytic activity of supported Au nanoparticles for the aerobic α-oxidation of amines to amides". Catal. Sci. Technol. 12 (6): 1922–1933. doi:10.1039/D1CY02121D. S2CID 246575960.

