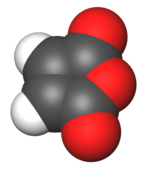
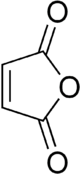Maleic anhydride
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Furan-2,5-dione
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.003.247 | ||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C4H2O3 | |||
| Molar mass | 98.06 g/mol | ||
| Appearance | White crystals | ||
| Density | 1.48 g/cm3 | ||
| Melting point | 52.8 °C (127.0 °F; 325.9 K) | ||
| Boiling point | 202 °C (396 °F; 475 K) | ||
| Reacts | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 102 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Maleic anhydride (cis-butenedioic anhydride, toxilic anhydride, dihydro-2,5-dioxofuran) is an organic compound with the formula C4H2O3. In its pure state it is a colourless or white solid with an acrid odour.
Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock:
- 2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O
Characteristic reactions
The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity.
- It hydrolyzes, producing maleic acid, cis-HO2CCH=CHCO2H. With alcohols, the half-ester is generated, e.g., cis-HO2CCH=CHCO2CH3.
- Maleic anhydride is a potent dienophile in Diels-Alder reactions.
- Maleic anhydride (MA) is an excellent ligand for low-valent metal complexes, examples being Pt(PPh3)2(MA) and Fe(CO)4(MA).
References
- ^ Merck Index, 11th Edition, 5586.
External links
- International Chemical Safety Card 0799
- NIOSH Pocket Guide to Chemical Hazards. "#0376". National Institute for Occupational Safety and Health (NIOSH).
- Chronic toxicity summary
- Maleic anhydride at Occupational Safety & Health Administration