McMurry reaction
| McMurry reaction | |
|---|---|
| Named after | John E. McMurry |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | mcmurry-reaction |

The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium(III) chloride and a reducing agent.[1] The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagent(s). Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium, zinc, and magnesium.[2][3] This reaction is related to the Pinacol coupling reaction which also proceeds by reductive coupling of carbonyl compounds.
Reaction mechanism
This reductive coupling can be viewed as involving two steps. First is the formation of a pinacolate (1,2-diolate) complex, a step which is equivalent to the pinacol coupling reaction. The second step is the deoxygenation of the pinacolate which yields the alkene, this second step exploits the oxophilicity of titanium.
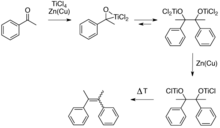
Several mechanisms have been discussed for this reaction.[4] Low-valent titanium species induce coupling of the carbonyls by single electron transfer to the carbonyl groups. The required low-valent titanium species are generated via reduction, usually with zinc powder. This reaction is often performed in THF because it solubilizes intermediate complexes, facilitates the electron transfer steps, and is not reduced under the reaction conditions. The nature of low-valent titanium species formed is varied as the products formed by reduction of the precursor titanium halide complex will naturally depend upon both the solvent (most commonly THF or DME) and the reducing agent employed: typically, lithium aluminum hydride,zinc-copper couple, zinc dust, magnesium-mercury amalgam, magnesium, or alkali metals.[5] Bogdanovic and Bolte identified the nature and mode of action of the active species in some classical McMurry systems,[6] and an overview of proposed reaction mechanisms has been published.[4] It is of note that titanium dioxide is not generally a product of the coupling reaction. Although it is true that titanium dioxide is usually the eventual fate of titanium used in these reactions, it is generally formed upon the aqueous workup of the reaction mixture.[5]
Background and scope
The original publication by Mukaiyama demonstrated reductive coupling of ketones using reduced titanium reagents.[7] McMurry and Fleming coupled retinal to give carotene using a mixture of titanium trichloride and lithium aluminium hydride. Other symmetrical alkenes were prepared similarly, e.g. civetone from adamantanone and tetraphenylethylene from benzophenone. A McMurry reaction using titanium tetrachloride and zinc is employed in the synthesis of a first-generation molecular motor.[8]
In another example, the Nicolaou's total synthesis of Taxol uses this reaction, although coupling stops with the formation of a cis-diol, rather than an olefin. Optimized procedures employ the dimethoxyethane complex of TiCl3 in combination with the Zn(Cu).
References
- ^ John E. McMurry; Michael P. Fleming (1974). "New method for the reductive coupling of carbonyls to olefins. Synthesis of β-carotene". J. Am. Chem. Soc. 96 (14): 4708–4709. doi:10.1021/ja00821a076. PMID 4850242.
- ^ Ian C. Richards "Titanium(IV) Chloride–Zinc" in Encyclopedia of Reagents for Organic Synthesis, John Wiley, London, 2001. doi:10.1002/047084289X.rt125. Article Online Posting Date: April 15, 2001
- ^ Martin G. Banwell, "Titanium(III) Chloride–Lithium Aluminum Hydride" in Encyclopedia of Reagents for Organic Synthesis John Wiley, London, 2001. doi:10.1002/047084289X.rt129. Article Online Posting Date: April 15, 2001
- ^ a b c Michel Ephritikhine (1998). "A new look at the McMurry reaction". Chem. Commun.: 2549–2554. doi:10.1039/a804394i.
- ^ a b Borislav Alois Furstner; Borislav Bogdanovic (1996). "New developments in the chemistry of low-valent titanium". Angew. Chem. Int. Ed. 35 (21): 2442–2469. doi:10.1002/anie.199624421.
- ^ Borislav Bogdanovic; Andreas Bolte (1995). "A comparative study of the McMurry reaction utilizing [HTiCl(THF)0.5]x, TiCl3(DME)1.5-Zn(Cu) and TiCl2*LiCl as coupling reagents". J. Organomet. Chem. 502: 109–121. doi:10.1016/0022-328X(95)05755-E.
- ^ Mukaiyama, T.; Sato, T.; Hanna, "Reductive Coupling of Carbonyl Compounds to Pinacols and Olefins by Using TiCl4 and Zn" Chem. Lett. 1973, 1041. doi:10.1246/cl.1973.1041
- ^ Matthijs K. J. ter Wiel, Richard A. van Delden, Auke Meetsma, and Ben L. Feringa (2003). "Increased Speed of Rotation for the Smallest Light-Driven Molecular Motor". J. Am. Chem. Soc. 125 (49): 15076–15086. doi:10.1021/ja036782o. PMID 14653742.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

