Mesoporous material
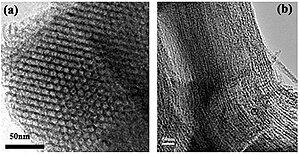
A mesoporous material is a material containing pores with diameters between 2 and 50 nm.
Porous materials are classified into several kinds by their size. According to IUPAC notation,[2] microporous materials have pore diameters of less than 2 nm and macroporous materials have pore diameters of greater than 50 nm; the mesoporous category thus lies in the middle.
Typical mesoporous materials include some kinds of silica and alumina that have similarly-sized fine mesopores. Mesoporous oxides of niobium, tantalum, titanium, zirconium, cerium and tin have also been reported. According to the IUPAC, a mesoporous material can be disordered or ordered in a mesostructure.
A procedure for producing mesoporous materials (silica) was patented around 1970.[3][4][5] It went almost unnoticed[6] and was reproduced in 1997.[7] Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan.[8] They were later produced also at Mobil Corporation laboratories [9] and named Mobil Crystalline Materials, or MCM-41.[10]
Since then, research in this field has steadily grown. Notable examples of prospective applications are catalysis, sorption, gas sensing, ion exchange, optics, and photovoltaics.
Mesopores may be defined differently in other contexts. For example, in the context of porous aggregations such as soil, mesopores are defined as cavities with sizes in the range 30 μm–75 μm.[11]
See also
References
- ^ Guo, M.; Wang, H.; Huang, D.; Han, Z.; Li, Q.; Wang, X.; Chen, J. (2014). "Amperometric catechol biosensor based on laccase immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/PVA matrix". Science and Technology of Advanced Materials. 15 (3): 035005. doi:10.1088/1468-6996/15/3/035005.
- ^ Rouquerol, J.; Avnir, D.; Fairbridge, C. W.; Everett, D. H.; Haynes, J. M.; Pernicone, N.; Ramsay, J. D. F.; Sing, K. S. W.; Unger, K. K. (1994). "Recommendations for the characterization of porous solids (Technical Report)". Pure and Applied Chemistry. 66 (8). doi:10.1351/pac199466081739.
- ^ Chiola, V.; Ritsko, J. E. and Vanderpool, C. D. "Process for producing low-bulk density silica." Application No. US 3556725D A filed on 26-Feb-1969; Publication No. US 3556725 A published on 19-Jan-1971
- ^ "Porous silica particles containing a crystallized phase and method" Application No. US 3493341D A filed on 23-Jan-1967; Publication No. US 3493341 A published on 03-Feb-1970
- ^ "Process for producing silica in the form of hollow spheres"; Application No. US 342525 A filed on 04-Feb-1964; Publication No. US 3383172 A published on 14-May-1968
- ^ Xu, Ruren; Pang, Wenqin and Yu, Jihong (2007). Chemistry of zeolites and related porous materials: synthesis and structure. Wiley-Interscience. p. 472. ISBN 0-470-82233-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Direnzo, F; Cambon, H; Dutartre, R (1997). "A 28-year-old synthesis of micelle-templated mesoporous silica". Microporous Materials. 10 (4–6): 283. doi:10.1016/S0927-6513(97)00028-X.
- ^ Yanagisawa, Tsuneo; Shimizu, Toshio; Kuroda, Kazuyuki; Kato, Chuzo (1990). "The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials". Bulletin of the Chemical Society of Japan. 63 (4): 988. doi:10.1246/bcsj.63.988.
- ^ Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W. (1992). "A new family of mesoporous molecular sieves prepared with liquid crystal templates". Journal of the American Chemical Society. 114 (27): 10834. doi:10.1021/ja00053a020.
- ^ Trewyn, B. G.; Slowing, I. I.; Giri, S.; Chen, H. T.; Lin, V. S. -Y. (2007). "Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release". Accounts of Chemical Research. 40 (9): 846–53. doi:10.1021/ar600032u. PMID 17645305.
- ^ Soil Science Glossary Terms Committee (2008). Glossary of Soil Science Terms 2008. Madison, WI: Soil Science Society of America. ISBN 978-0-89118-851-3.
