Metal-formaldehyde complex
This article may be too technical for most readers to understand. (March 2024) |
A metal-formaldehyde complex is a coordination complex in which a formaldehyde ligand has two bonds to the metal atom(s) (η2-CH2O). This type of ligand has been reported in both monometallic and bimetallic complexes.
History
[edit]Metal-formaldehyde complexes have been reported for tungsten (W), osmium (Os),[1] vanadium (V),[2] rhenium (Re),[3] zirconium (Zr),[4][5][6] ruthenium (Ru),[7] and niobium (Nb).[8]
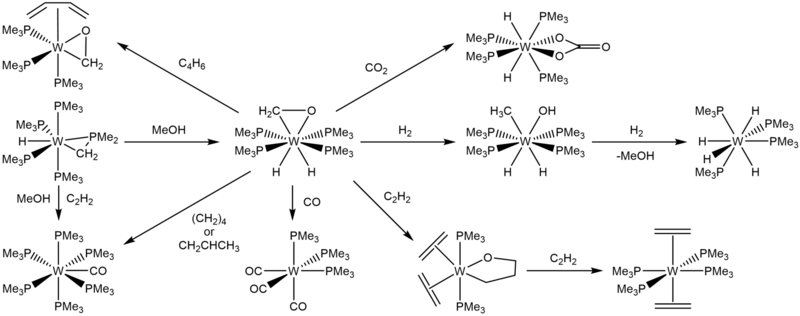
In 1984, Green and coworkers reported the yellow crystalline solid W(PMe3)4(η2-CH2O)H2. It was the result of the addition of methanol to W(PMe3)4(η2-CH2PMe2)H.
W(PMe3)4(η2-CH2O)H2 can be hydrogenated to give W(PMe3)4(MeO)H3, and then further hydrogenated to reform methanol and generate W(PMe3)4H4.[9] In 1986, Green and Parkin demonstrated further reactivities of W(PMe3)4(η2-CH2O)H2. Upon addition of CO or CO2, W(PMe3)4(η2-CH2O)H2 produces fac-W(PMe3)3(CO)3 and W(PMe3)4(κ2-O2CO)H2, respectively, much like its precursor.
W(PMe3)4(η2-CH2O)H2 also reacts with buta-1,3-diene to give W(PMe3)3(η2-CH2O)(η-C4H6).[10]
W(PMe3)4(η2-CH2O)H2 can also be used as a route to further oxometallacycles by the addition of ethylene and rapid cooling to –80°C. The resultant green-colored crystals are composed of W(OCH2CH2CH2)(PMe3)2(η2-C2H4)2, with either both ethylene ligands on the equatorial plane or the ethylene ligand cis- to the ligating oxygen in the axial direction. Further reaction with ethylene produces trans-W(PMe3)4(η2-C2H4)2 and W(PMe3)4(CO)H2.[10]
References
[edit]- ^ Clark, G.R.; Headford, C.E.L.; Marsden, K.; Roper, W.R. (June 1982). "Synthesis, structure and reactions of a dihapto-formaldehyde complex, Os(η2-CH2O)(CO)2(PPh3)2". Journal of Organometallic Chemistry. 231 (4): 335–360. doi:10.1016/s0022-328x(00)81212-7. ISSN 0022-328X.
- ^ Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (April 1982). "Metal-formaldehyde chemistry: coordination, disproportionation and Lewis acid-promoted transformation to oxymethylene derivatives". Journal of the American Chemical Society. 104 (7): 2019–2020. doi:10.1021/ja00371a038. ISSN 0002-7863.
- ^ Buhro, William E.; Patton, Alan T.; Strouse, Charles E.; Gladysz, J. A.; McCormick, Fred B.; Etter, Margaret C. (February 1983). "Syntheses, chemical properties, and x-ray crystal structures of rhenium formaldehyde and thioformaldehyde complexes". Journal of the American Chemical Society. 105 (4): 1056–1058. doi:10.1021/ja00342a070. ISSN 0002-7863.
- ^ Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (March 1983). "Genesis, bonding mode and reaction with carbon monoxide of an oxymethylene unit bridging two metal atoms". Journal of the American Chemical Society. 105 (6): 1690–1691. doi:10.1021/ja00344a066. ISSN 0002-7863.
- ^ Kropp, Kurt; Skibbe, Volker; Erker, Gerhard; Krueger, Carl (May 1983). "Fischer-Tropsch intermediates: tris[(.eta.2-formaldehyde)zirconocene] from the carbonylation of a zirconium hydride". Journal of the American Chemical Society. 105 (10): 3353–3354. doi:10.1021/ja00348a075. ISSN 0002-7863.
- ^ Fachinetti, Giuseppe; Floriani, Carlo; Pucci, Sergio (1978-01-01). "Stoicheiometric reduction of CO and CO2 to methanol: evidence for carbon monoxide insertion into zirconium–hydrogen bond". Journal of the Chemical Society, Chemical Communications (6): 269–270. doi:10.1039/C39780000269. ISSN 0022-4936.
- ^ Chaudret, Bruno N.; Cole-Hamilton, David J.; Nohr, Ronald S.; Wilkinson, Geoffrey (1977-01-01). "The reactions of chlorohydrido- and dichloro-tris(triphenylphosphine)ruthenium(II) with alkali hydroxides and alkoxides. Hydridohydroxobis(triphenylphosphine)ruthenium(II) monosolvates, their reactions and related compounds". Journal of the Chemical Society, Dalton Transactions (16): 1546–1557. doi:10.1039/DT9770001546. ISSN 1364-5447.
- ^ Wolczanski, Peter T.; Threlkel, Richard S.; Bercaw, John E. (January 1979). "Reduction of coordinated carbon monoxide to "zirconoxy" carbenes with permethylzirconocene dihydride". Journal of the American Chemical Society. 101 (1): 218–220. doi:10.1021/ja00495a037. ISSN 0002-7863.
- ^ Green, Malcolm L. H.; Parkin, Gerard; Moynihan, Kelly J.; Prout, Keith (1984-01-01). "Formation of an η2-formaldehyde compound from methanol and its hydrogenation giving methanol". Journal of the Chemical Society, Chemical Communications (22): 1540. doi:10.1039/C39840001540. ISSN 0022-4936.
- ^ a b Green, Malcolm L. H.; Parkin, Gerard (1986-01-01). "Ethylene insertion into the W–C bond of the η2-formaldehyde ligand system of W(PMe3)4(η2-CH2O)H2 giving the oxometallacyclopentane derivative W(OCH2CH2CH2)(PMe3)2(C2H4)2 and related studies". Journal of the Chemical Society, Chemical Communications (1): 90–91. doi:10.1039/C39860000090. ISSN 0022-4936.
