Methane (data page)
Appearance
This page provides supplementary chemical data on methane.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as Iowa State, and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | ? |
| Dielectric constant, εr | 1.7 ε0 at ? °C |
| Bond strength | ? |
| Bond length | 0.117 nm |
| Bond angle | 109.5 |
| Magnetic susceptibility | −17.4×10−6 cm3/mol |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 90.67 K (−182.48 °C), 0.117 bar |
| Critical point | 190.6 K (−82.6 °C), 46 bar |
| Std enthalpy change of fusion, ΔfusH |
1.1 kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
8.17 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
−73.4 kJ/mol[1] |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 52.93 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−74.87 kJ/mol |
| Standard molar entropy, S |
186.61 J/(mol K) |
| Enthalpy of combustion ΔcH |
−882.0 kJ/mol |
| Heat capacity, cp | 35.69 J/(mol K) |
| van der Waals' constants[2] | a = 228.29 L2 kPa/mol2 b = 0.04278 liter per mole |
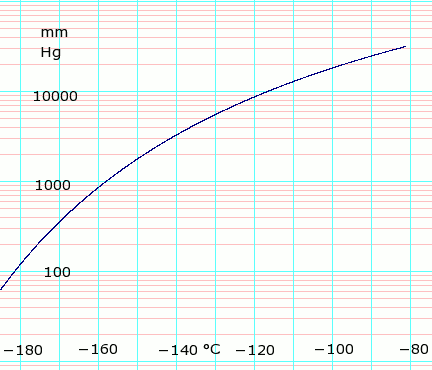
Vapor pressure of liquid
| P (mm Hg) | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T (°C) | −205.9(s) | −195.5(s) | −187.7(s) | −181.4 | −168.8 | −161.5 | −152.3 | −138.3 | −124.8 | −108.5 | −86.3 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Annotation "(s)" indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. Note that these are all negative temperature values.
Spectral data

| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ~3020 cm−1 |
| ~1300 | |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
References
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.

