Moschorhinus
| Moschorhinus Temporal range: Late Permian-Early Triassic
| |
|---|---|
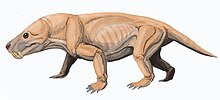
| |
| Restoration | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Synapsida |
| Clade: | Therapsida |
| Clade: | †Therocephalia |
| Family: | †Akidnognathidae |
| Genus: | †Moschorhinus Broom, 1920 |
| Type species | |
| †Moschorhinus kitchingi Broom, 1920
| |
Moschorhinus is an extinct genus of therocephalian of the Akidnognathidea family. It was a carnivorous quadruped and lived primarily in the Permian period. Described by South African paleontologist Robert Broom in 1920, its name is derived from the Ancient Greek words μόσχος (mos'-khos) moschos for calf and rhino- for nose, referring to the broad, blunt shape of the snout.
Its short strong skull bore long straight canines and was up to lion-sized. It resembled the gorgonopsids, whose predatory role it appears to have replaced.[1]
While most abundant in the late Permian, remains of Moschorhinus kitchingi have also been found in the earliest Triassic beds in the Karoo basin, showing that Moschorhinus did survive the Permian-Triassic extinction event, but disappeared soon afterwards.[2][3] Although smaller than their Permian predecessors, Triassic Moschorhinus were the largest therocephalian predators of their time.[3][4] An examination of the change in size between Moschorhinus fossils from before and after the mass extinction at the end of the Permian provides an excellent study of the Lilliput effects observable in species known to have survived an environmental catastrophe such as an extinction event.
Etymology
Its name is derived from the Ancient Greek words μόσχος (mos'-khos) moschos for calf or young animal, and rhin/rhino- for nose or snout. The species name, kitchingi, refers to Mr. James Kitching, who originally found (but did not describe) the specimen.[5]
Discovery
The first Moschorhinus specimen was discovered by Mr. James Kitching in the Karoo Beds of South Africa, near a New Bethesda Road. It was first described by paleontologist Robert Broom in 1920, who published his observations through the Zoological Society of London.[5]
Environment
Location
Many Therocephalian remains have been discovered in rocks in the Karoo Basin of South Africa from the Mid Permian through Mid Triassic assemblages. Moschorhinus remains have been found most prominently in the Beaufort Group of the Karoo Basin. They are often found very close to remains of the herbivorous dicynodonts.[3][6] Specifically, remains of Moschorhinus kitchingi and many therocepahlians are uncovered in the Upper Permian Dicynodon (DAZ) and lower Triassic Lystrosaurus (LAZ) assemblage zones in the Karoo Basin of South Africa.[6][7]
The above figure shows the stratigraphy and geographic distributions of Moschorhinus in the Beaufort Group of the Balfour Formation in the Karoo Basin of South Africa. Specimens found in rock from the Upper Permian are shown in dark gray, and lowermost Triassic in light gray.[6]
Stratigraphic
Many vertebrate fossils have been uncovered in the Karoo Basin of South Africa in rock from the Upper Permian to Lower Triassic levels.[8] Here, remains of therocephalian theraspids have been found on both sides of the Permo-Triassic boundary; the therocephalian genera Tetracynodon, Promoschorhynchus, and Moschorhinus have all been revealed in both biostratigraphic levels.(1) Within this order, Moschorhinus specimens were the only large predators found in both biostratigraphic strata.[4][6]
The first appearances of Moschorhinus is debatable, either occurring first in the upper Cistecephalus (CiAZ) or Lower Dicynodon (DAZ) Assemblage Zones of the Karoo basin, both of the late Permian. Remains have been widely found and described from the very uppermost Permian, also from the DAZ.[3][6]
At the uppermost scope, Moschorhinus has recently been encountered in rocks of the Balfour Formation dating to the Early Triassic. However, despite concentrated efforts, the genus has not been found in the Katberg Formation of Triassic rock.[3] Based on the fossil record thus far, Lystrosaurus, Moschorhinus and Owenetta all became extinct at the beginning of the sedimentation of this formation.[8] Thus, the latest observed appearance of Moschorhinus occurs in the lower LAZ.
The mass extinction at the end of the Permian cued the extinction of 80-95% of animal species. Based on models of sediment burial, weathering and oxidation of organic matter, scientists have been able to deduce a gradual decrease in atmospheric oxygen ratios across the Permo-Triassic boundary. It is estimated that oxygen was present as 30% of the atmospheric makeup of the Early Permian, but reduced to just 15% by the Early Triassic. Physiological studies show that this induced hypoxia likely had a negative effect on the potential body sizes of tetrapods of the time.[3] In addition to the transition from hyperoxic to hypoxic conditions, animals of the Early Triassic faced intense seasonal climate changes, reduced ecosystem diversity, and a loss of forested land. These populations were thus very susceptible to effects of environmental variability, as this type of unpredictable environment can result in drastic disturbances to primary plant production. This also resulted in lowered herbivore abundance, which would have had a major influence on predators of the early Triassic, Moschorhinus included.[6]
Description
Cranial anatomy

The therocephalian Moschorhinus is unusually recognizable based on its skull. The skull is similar to that of the Gorgonopsids, with convergently evolved cranial features. Moschorhinus skulls feature large temporal fenestra (three in total, as it is a synapsid) and a convexly bowed palate, and range in size from skulls of a monitor lizard, to those of a lion. As referenced in the name—“moscho” for calf, and “rhinus” for nose—they possess a characteristically short, broad snout, which houses a curving set of laterally compressed incisors. Most prominently recognizable, are the large, straight, round incisors, similar to the design of a saber-toothed cat. These formidable teeth are accompanied by multiple palatal fenestrae, in order to house the muscular attachments necessary for such a bite.[1]
Lateral view of the skull of Moschorhinus (redrawn from Durand, 1991) [9]
Lateral view of Moschorhinus jaw, showing range of motion necessary for such large incisors, and upper palatal fenestrae of snout. (From van Valkenburgh and Jenkins, 2002).[1]
Snout
The snout of Moschorhinus is characteristically short and broad, lending itself to the creation of the name, which literally translates to “calf-nosed,” taken from the Greek “Moscho” and “rhin.” At the most anterior/rostral end of the upper jaw, the blunt snout features a medial frontonasal ridge, best seen in the diagram above[1] illustrating the palatal view of the upper jaw.[6] The anterior end of the lower jaw is characterized by an expanded symphysis, the seam where the two dentaries join together, which is much larger than that of any other therocephalian.[1] The dentary bones housing the teeth of the lower jaw are thick and sturdy, each expanded laterally.[6] On the upper jaw, the premaxillary bones are broad and strong, each housing 6 large incisors, smooth and mostly flattened.[5] On its anterior dorsal edge, the premaxilla projects over the incisors, forming a rostral process, however this is only displayed in juveniles, and is reversed in adult Moschorhinus. The maxilla is short and strong.[6] Part of the maxilla overlaps the premaxilla. It houses one large canine, and a reduced number of teeth posterior to the canine. Based on indentations in the maxilla, it is presumed that there were three molars, the third being very small. In total this pattern is described as a dental formula of I6.C1.M3, referring to the 6 incisors, 1 canine, and 3 molars seen symmetrically on either side of the upper jaw.[5] The septomaxilla is large, and also overlaps the premaxilla on its anterior end.[6] It does not overlap between the maxilla and nasal bones, and forms a large portion of the perimeter of the external nostril. There is a small, reduced foramen between the septomaxilla and maxilla, understandable given the strength needed to pierce prey with such large canines.[5] The external nares (nostrils) are large, and positioned more on the anterior facing plane of the snout than they are lateral facing, bringing them closer together on the front edge of the snout.[6]
Teeth
Beginning with the teeth on the most mesial/rostral end of the upper jaw, first are the incisors; each premaxilla, shallowly curving, houses six. The incisors are enlarged and laterally compressed, described as having a bell-shaped cross-section. Their external surfaces, on the mesial (facing cheek) side, are smooth cutting edges, and unlike other therocephalians, lack any fluting or facets/striae.[6] The large canines are a quickly identifiable feature of Moschorhinus. They are uniquely rounded in cross-section, and especially thick and strong. In length, these sabers are comparable to almost all of the gorgonopsids. While there is no modern animal with canines akin to those of Moschorhinus, the most similar living example would be the Clouded Leopard (Neofelis nebulosa), who also shares the feature of greatly elongated canines. Based on the shallow, flat incisors and long, durable canines, it is presumed that Moschorhinus was a predator with a cat-like mode of bringing down its prey, being able to bite clean cuts, and have the skeletal capacity and strength to endure piercing and holding on to its prey with its canines. Based on dental arrangements, this is the first known development of a design lending itself to this mode of predation. Nonetheless, given its sturdily designed, thick snout, enormous canines, and powerful jaw muscles, the teeth of Moschorhinus show it to have been a daunting predator.[1] A feature shared with close relatives, Moschorhinus shows a reduction of the number of teeth posterior to its canines. Each maxilla is short, and along with the canines, held 3 molars. (This pattern has been inferred by the indentations left on the bone). In most therocephalians, the “teeth,” or rather denticulation of the pterygoids, are greatly reduced or missing, and in Moschorhinus they are absent.[6][10]
Skull roof and temporal faces
The genus Moschorhinus falls into the Synapsid clade. This clade includes all taxa (both extinct and extant) which are more closely related to the crown group mammals than to crown reptiles. As a group, synapsid skulls possess a lateral temporal fenestra, as seen in Moschorhinus.[8] Tracing the roof of the skull, Moschorhinus possess small prefrontal bones, followed by large, widened frontal bones. They do not feature a postfrontal. The parietals form a narrow crest, which is extended forwards in adult specimens, and houses a very basic pineal foramen.[5][6] The parietals are also one of the dermal elements making up the braincase, forming a crest over the top of the brain case and temporal fossa. The walls of the temporal fossa are completed by the squamosal, epipterygoid, pterygoid, and prootic bones, as well as part of the opisthotic, supraoccipital and interparietal bones. Indentations can be seen in the temporal fossa indicating the presence of many blood vessels and nerves supplying the brain.[11]
Orbit
The lacrimal bone is larger than the reduced prefrontal, and forms the majority of the orbital margin. The lacrimal has a bony boss (a rounded knob) on the orbit, and a large foramen towards its inner side. Forming the lower edge of the orbit, a deep and reinforced suborbital arch is produced by the jugal and maxillary bones.[5] The anterior length of the jugal ends at the anterior edge of the orbit, and is not convex, as in several later genera.[6]
Palate
In Broom’s initial description of Moschorhinus, the palate was the best preserved part of his specimen. In general, the palate is structurally similar to that of akidognathus, though proportioned differently.[5] Overall, the palate is convex, with a broad, triangular vomer, with paired tubercles, rounded projections pointing ventrally.[1][6] The palatines are enlarged and thick, especially on their outer (labial) edges where they articulate with the maxilla. On their inner (lingual) edges, the palatines articulate with the pterygoid and prevomer, and posteriorly form part of the circumference of the suborbital vacuity. Between the palatine and maxilla, just behind the canines, are large foramens; these foramens correspond to similar structures in akidognathus, so are presumably to allow for nerves. A slanting ridge along the middle of the palatine presumably supported a soft palate, allowing the internal nares to carry back into a choana in the pterygoid region.[1] The prevomers are stiffened and bound anteriorly, and expand to form a widened shield between the internal nares. Here they are constricted and more narrow, but closer to the pterygoids they widen out to a more triangular shape between the palatines. Here, a clear suture can be seen medially between the prevomers.[1] Flanking the pterygoids, the irregularly shaped ectopterygoids articulate on their anterior side with the palatines, and on their posterior side with the pterygoid. Here, they enlarge to form a process in front of the pterygoid process. Labially, the ectopterygoids meet with the maxilla and jugal bones, and between each maxilla and ectopterygoid, there is a small foramen.[5] There are no pterygoid vacuities observed in the palate of Moschorhinus, and the projection of the pterygoid is rounded to a concave form, possibly carrying swallowing muscles, and does not house teeth.[5][10] The genus Promoschorynchus, a close relative of Moschorhinus, shares the form of dagger-like canines. While excellent for hunting, this feature requires the mouth to open widely for use, making feeding difficult. There is not sufficient support from soft tissue impressions of Moschorhinus, but Promoschorynchus showed stiff choanal folds used to maintain the opening of the air passage in order to allow for breathing while ingesting food, and it is possible that this feature was present in Moschorhinus. The development of a secondary palate in the skull was seen gradually across the span of therocephalians, and the choanal crest is featured in all later therocephalians.[10]
Lilliput effects
The Lilliput effect is a term coined in 1993, used to describe the dramatic reduction in size observed in populations of a taxa which have survived a major extinction event, attributable to a variety of environmental factors.[12] This effect has been documented in numerous “survivor” taxa of such events. In his work, Adam K. Huttenlocker examined fossil records of Moschorhinus kitchingi from before and after the mass extinction demarcating the Permo-Triassic boundary. Specifically, he looked at changes in cranial size and limb bone histology indicating vascular growth patterns. Great size reductions are observed in Triassic Moschorhinus, as compared to their Permian forerunners. Histological evidence shows that Permian and Triassic Moschorhinus skeletons grew at different rates early in life, largely believed to be an effect of the harsher environmental variability of the early Triassic. This less stable environment favored a faster growth and development to reach a minimum body size requirement. Because of the Lilliput effects observed across the Permo-Triassic boundary, both size and stratigraphic stage must be considered when determining the maturity and age (in life) of a specimen.[3]
Evidence from cranial sizes
Triassic Moschorhinus skulls were found to be significantly smaller, with decreased basal skull lengths (BSL) from those of the Permian specimens. Permian skulls’ BSL measurements averaged 207mm, while the mean Triassic skull was only 179mm.[3]
Evidence from bone histology
Analysis of histological bone studies suggest an accelerated development in Triassic Moschorhinus as compared to those of the Permian.[3] Limb bones more perforated for vascularization, and with fewer growth marks, and thickened growth zones are indicative of faster, earlier growth rates, and are found in the Triassic specimens, whereas Permian specimens of equal sizes showed radial growth and addition of vascularization, and more growth marks, indicating slower growth over a longer age. Given a more stable environment, Permian Moschorhinus has the luxury of a cyclic growth pattern over several years, accelerating or slowing with multiple growing seasons before reaching a larger, adult size. Triassic individuals, although smaller in size, showed histological indications of very rapid, uninterrupted growth over a shorter time, with especially accelerated growth rates at early ages.[6] This favors the theory that the turbulent post-crisis conditions of the early Triassic created a selective partiality towards rapid, young development to a near-mature size in a shorter time.[3] Overall, these histological differences across the time periods reflect the fact that size alone cannot be used as a reliable measure of age and maturity of Moschorhinus specimens, without cross-referencing the relative geological time from which they were recovered. Because the post-crisis environment of the early Triassic featured limited resources, lowered primary production, and newly hypoxic conditions, rapid growth early in life to a minimum body size was a biologically selected adaptation that proved best suited for populations in the early Triassic environments, despite its seemingly costly energy investment, when compared to the prolonged growth patterns seen in the Permian, more suited to stable resources and conditions.[3]
Phylogeny and classification
As mentioned, Moschorhinus are part of the clade Synapsida, including all extinct and extant taxa more closely related to crown group (modern living) mammals than they are to crown group reptiles. As a primitive condition, this clade features a single lateral fenestra.[8] Moschorhinus fall into the order Theraspida, and are often also referred to as “mammal-like reptile,” as they show transitional features shared by both the named clades. Suborders used to describe this genus include Eutheriodontia and Therocephalia. The name Therocephalia refers to the large skulls of this suborder and their large, carnivorous-looking teeth, and means, “beast headed.” The family containing Moschorhinus is Akidnognathidae, large, carnivorous theraspids all sharing strong skulls and large upper canines. Together they form the intermediate leading to the morphological derivation of Bauriodea. Only one species has been identified within the genus Moschorhinus, this is Moschorhinus kitchingi.
While living, Moschorhinus occupied a similar niche as gorgonopsids, who faced extinction at the end of the Permian. Morphologically, the two groups were similar, each designed as formidable, felid-like predators. Given their similarity in morphology and proposed ecological niche, it is at present still unclear what feature of Moschorhinus allowed it to out last the Gorgonopsids into the early Triassic. Following the extinction of Moschorhinus, cynodonts took over a similar niche, filling the role of top predators as the Triassic moved forward.[1]
References
- ^ a b c d e f g h i j van Valkenburgh B, Jenkins I (2002). "EVOLUTIONARY PATTERNS IN THE HISTORY OF PERMO-TRIASSIC AND CENOZOIC SYNAPSID PREDATORS" (PDF). Paleontological Society Papers. 8: 267–88. Archived from the original (PDF) on 2013-10-17.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Botha J, Smith RM (2006). "Rapid vertebrate recuperation in the Karoo Basin of South Africa following the End-Permian extinction" (PDF). Journal of African Earth Sciences. 45 (4–5): 502–14. doi:10.1016/j.jafrearsci.2006.04.006. [dead link]
- ^ a b c d e f g h i j k Huttenlocker AK, Botha-Brink J (2013). "Body size and growth patterns in the therocephalian Moschorhinus kitchingi (Eutheriodontia) before and after the end-Permian extinction in South Africa". Paleobiology. 39 (2): 253–77. doi:10.1666/12020.
- ^ a b Christian A. Sidor; Roger M. H. Smith; Adam K. Huttenlocker; Brandon R. Peecook (2014). "New Middle Triassic Tetrapods from the Upper Fremouw Formation of Antarctica and Their Depositional Setting". Journal of Vertebrate Paleontology. 34 (4): 793–801. doi:10.1080/02724634.2014.837472.
- ^ a b c d e f g h i j Broom R (1920). "On Some New Therocephalian Reptiles from the Karroo Beds of South Africa". Proceedings of the Zoological Society of London: 351–354.
- ^ a b c d e f g h i j k l m n o p q Huttenlocker, Adam (2013). The Paleobiology of South African Therocephalian Therapsids (Amniota, Synapsida) and the Effects of the End-Permian Extinction on Size, Growth, and Bone Microstructure (Ph.D). University of Washington.
- ^ Rubidge Bruce S, Sidor Christian A (2001). "EVOLUTIONARY PATTERNS AMONG PERMO-TRIASSIC THERAPSIDS". Annual Review of Ecology and Systematics. 32: 449–480. doi:10.1146/annurev.ecolsys.32.081501.114113.
- ^ a b c d Peter D Ward; Jennifer Botha; Roger Buik; Michiel O. De Kock; Douglas H. Erwin; Geoffrey H Garrison; Joseph L Kirschvink; Roger Smith (2005). "Abrupt and Gradual Extinction Among Late Permian Land Vertebrates in the Karoo Basin, South Africa". Science. 307 (5710): 709–714. doi:10.1126/science.1107068. PMID 15661973.
- ^ Huttenlocker Adam (2009). "An Investigation into the Cladistic Relationships and Monophyly of Therocephalian Theraspids". Zoological Journal of the Linnean Society. 157 (4): 865–891. doi:10.1111/j.1096-3642.2009.00538.x.
- ^ a b c Maier W, van den Heever J, Durand F (1996). "New therapsid specimens and the origin of the secondary hard and soft palate of mammals". Journal of Zoological Systematics and Evolutionary Research. 34: 9–19. doi:10.1111/j.1439-0469.1996.tb00805.x.
- ^ Durand J F (1991). "A revised descripction of the skull of moschorhinus (therapsida, therocephalia)". Annals of the South African Museum. 99: 381–413.
- ^ Richard J Twitchett (2007). "The Lilliput effect in the aftermath of the end-Permian extinction event" (PDF). Palaeogeography, Palaeoclimatology, Palaeoecology. 252: 132–144. doi:10.1016/j.palaeo.2006.11.038.



