Newman projection
| Molecule of butane in syn-clinal (-sc) conformation | ||
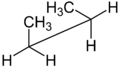
|

|
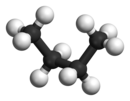
|
| Sawhorse projection |
Newman projection |
3D structure |
A Newman projection, useful in alkane stereochemistry, visualizes the conformation of a chemical bond from front to back, with the front atom represented by a dot and the back carbon as a circle. The front carbon atom is called proximal, while the back atom is called distal. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms.[1]
This diagram style is an alternative to a sawhorse projection, which views a carbon-carbon bond from an oblique angle, or a wedge-and-dash style, such as a Natta projection. These other styles can indicate the bonding and stereochemistry, but not as much conformational detail.
This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as partial replacement for Fischer projections, which are unable to represent conformations and thus conformers properly.[2][3]
A Newman projection can be used to visualize any sort of bond, not just a single bond between carbons of an alkane. For example, it can be used to study cyclic molecules,[2] such as the chair conformation of cyclohexane:

|
File:C6 Newman.svg | 
|
| Bond-line structure | Newman projection | 3D structure |
See also
References
- ^ Moss, GP (1996-01-01). "Basic terminology of stereochemistry (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (12): 2193–2222. doi:10.1351/pac199668122193. ISSN 1365-3075.
- ^ a b Newman, MS (1955). "A notation for the study of certain stereochemical problems". Journal of Chemical Education. 32 (7): 344. doi:10.1021/ed032p344. ISSN 0021-9584.
- ^ Newman, MS. Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952, 13, 111
