Nickel sulfide
| |
| Names | |
|---|---|
| Preferred IUPAC name
Nickel sulfide [1] | |
| Other names
nickel monosulfide, nickelous sulfide
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.037.113 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| NiS | |
| Molar mass | 90.7584 g mol−1 |
| Appearance | black solid |
| Odor | Odorless |
| Density | 5.87 g/cm3 |
| Melting point | 797 °C (1,467 °F; 1,070 K) |
| Boiling point | 1,388 °C (2,530 °F; 1,661 K) |
| insoluble | |
| Solubility | soluble in nitric acid |
| Structure | |
| hexagonal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
may cause cancer by inhalation |
| GHS labelling: | |

| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nickel sulfide is the inorganic compound with the formula NiS. It is a black solid that is produced by treating nickel(II) salts with hydrogen sulfide. Many nickel sulfides are known, including the mineral millerite, which also has the formula NiS. Aside from being useful ores, nickel sulfides are the products of desulfurization reactions, and are sometimes used as catalysts. Nonstoichiometric forms of nickel sulfide are known, e.g., Ni9S8 and Ni3S2.
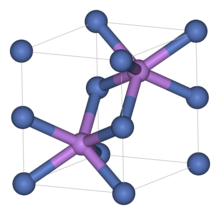
Structure
Like many related materials, nickel sulfide adopts the nickel arsenide motif. In this structure, nickel is octahedral and the sulfide centers are in trigonal prismatic sites.[2]
Nickel sulfide has two polymorphs. The alpha form has a hexagonal unit cell, while the beta form has a rhombohedral cell. Small amounts of this compound occur as imperfection when glass is manufactured. It is believed that cracks occur in toughened window glass panels due to the volume change associated with the alpha to beta phase transition of these nickel sulfide defects.[3][4]
Synthesis
The precipitation of solid black nickel sulfide is a mainstay of traditional qualitative inorganic analysis schemes, which begins with the separation of metals on the basis of the solubility of their sulfides. Such reactions are written:[5]
- Ni2+ (aq) + H2S (aq) → NiS (s) + 2 H+ (aq)
Many other more controlled methods have been developed, including solid state metathesis reactions (from NiCl2 and Na2S) and high temperature reactions of the elements.[6]
Occurrence
The mineral millerite is also a nickel sulfide with the molecular formula NiS, although its structure differs from synthetic stoichiometric NiS due to the conditions under which it forms. It occurs naturally in low temperature hydrothermal systems, in cavities of carbonate rocks, and as a byproduct of other nickel minerals.[7]

References
- ^ "Nickel sulfide - Compound Summary". Retrieved October 17, 2012.
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Bishop, D.W.; Thomas, P.S.; Ray, A.S. (1998). "Raman spectra of nickel(II) sulfide". Materials Research Bulletin. 33 (9): 1303. doi:10.1016/S0025-5408(98)00121-4.
- ^ "NiS and Spontaneous Breakage". Glass on Web. Nov 2012.
- ^ O.Glemser "Nickel Sulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 2. p. 1551.
- ^ leading reference can be found in: Shabnam Virji, Richard B. Kaner, Bruce H. Weiller "Direct Electrical Measurement of the Conversion of Metal Acetates to Metal Sulfides by Hydrogen Sulfide" Inorg. Chem., 2006, 45 (26), pp 10467–10471.doi:10.1021/ic0607585
- ^ Gamsjager H. C., Bugajski J., Gajda T., Lemire R. J., Preis W. (2005) Chemical Thermodynamics of Nickel, Amsterdam, Elsevier B.V.
