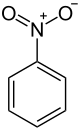
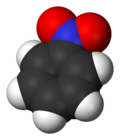
Nitrobenzene
| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
Nitrobenzol
Oil of mirbane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.469 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.06 g/mol | ||
| Appearance | yellowish, oily liquid[1] | ||
| Odor | pungent, like paste shoe polish[1] | ||
| Density | 1.199 g/cm3 | ||
| Melting point | 5.7 °C (42.3 °F; 278.8 K) | ||
| Boiling point | 210.9 °C (411.6 °F; 484.0 K) | ||
| 0.19 g/100 ml at 20 °C | |||
| Vapor pressure | 0.3 mmHg (25°C)[1] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 88 °C (190 °F; 361 K) | ||
| 480 °C (896 °F; 753 K) | |||
| Explosive limits | 1.8%-?[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
780 mg/kg (rat, oral) 600 mg/kg (rat, oral) 590 mg/kg (mouse, oral) [2] | ||
LDLo (lowest published)
|
750 mg/kg (dog, oral)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 ppm (5 mg/m3) [skin][1] | ||
REL (Recommended)
|
TWA 1 ppm (5 mg/m3) [skin][1] | ||
IDLH (Immediate danger)
|
200 ppm[1] | ||
| Related compounds | |||
Related compounds
|
Aniline Benzenediazonium chloride Nitrosobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.
Production
Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = −117 kJ/mol).[3]
World capacity for nitrobenzene in 1985 was about 1.7×106 tonnes.[3]
Mechanism of nitration
The nitration process involves formation of the nitronium ion (NO2+), followed by an electrophilic aromatic substitution reaction of it with benzene. The nitronium ion is generated in situ by the reaction of nitric acid and an acidic dehydration agent, typically sulfuric acid:
- HNO3 + H+ ⇌ NO2+ + H2O
Uses
Approximately 97% of nitrobenzene is consumed in the production of aniline,[3] which is a precursor to rubber chemicals, pesticides, dyes (particularly azo dyes), explosives, and pharmaceuticals.
Specialized applications
Nitrobenzene is also used to mask unpleasant odors in shoe and floor polishes, leather dressings, paint solvents, and other materials. Redistilled, as oil of mirbane, nitrobenzene has been used as an inexpensive perfume for soaps. A significant merchant market for nitrobenzene is its use in the production of the analgesic paracetamol (also known as acetaminophen) (Mannsville 1991).[4] Nitrobenzene is also used in Kerr cells, as it has an unusually large Kerr constant.
Organic reactions
Aside from its conversion to aniline, nitrobenzene is readily converted to related derivatives such as azobenzene,[5] nitrosobenzene,[6] and phenylhydroxylamine.[7] The nitro- group is deactivating, thus substitution tends to occur at the meta-position.
Safety
Nitrobenzene is highly toxic (Threshold Limit Value 5 mg/m3) and readily absorbed through the skin.
Prolonged exposure may cause serious damage to the central nervous system, impair vision, cause liver or kidney damage, anemia and lung irritation. Inhalation of vapors may induce headache, nausea, fatigue, dizziness, cyanosis, weakness in the arms and legs, and in rare cases may be fatal. The oil is readily absorbed through the skin and may increase heart rate, cause convulsions or rarely death. Ingestion may similarly cause headaches, dizziness, nausea, vomiting and gastrointestinal irritation, loss of sensation/use in limbs and also causes internal bleeding.[6]
Nitrobenzene is considered a likely human carcinogen by the United States Environmental Protection Agency,[8] and is classified by the IARC as a Group 2B carcinogen which is "possibly carcinogenic to humans".[9] It has been shown to cause liver, kidney, and thyroid adenomas and carcinomas in rats.[10]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[11]
Popular culture
- The 1927 short story The Avenging Chance by Anthony Berkeley discusses contemporary uses of nitrobenzene.
- In the 1937 Nero Wolfe detective novel The Red Box by Rex Stout, a person is murdered by having oil of mirbane spilled on him in his car.
References
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0450". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Nitrobenzene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Gerald Booth (2007). "Nitro Compounds, Aromatic". In: Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons: New York. doi:10.1002/14356007.a17_411
- ^ Bhattacharya A.; Purohit V. C.; Suarez, V.; Tichkule, R; Parmer, G.; Rinaldi, F. (2006). "One-step reductive amidation of nitro arenes: application in the synthesis of Acetaminophen". Tetrahedron Letters. 47 (11): 1861–1864. doi:10.1016/j.tetlet.2005.09.196.
- ^ Bigelow, H. E.; Robinson, D. B. (1955). "Azobenzene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 103. - ^ a b G. H. Coleman, C. M. McCloskey, F. A. Stuart. "Nitrosobenzene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 668. - ^ O. Kamm. "β-Phenylhydroxylamine". Organic Syntheses; Collected Volumes, vol. 1, p. 445.
- ^ http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickView&substance_nmbr=0079
- ^ Agents Classified by the IARC Monographs, International Agency for Research on Cancer
- ^ National Institutes of Health · U.S. Department of Health and Human Services, Nomination: Nitrobenzene Review committee, 02/02/2010
- ^ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: postscript (link)