Nornicotine
Appearance

| |
| Names | |
|---|---|
| IUPAC name
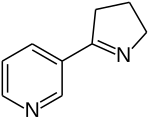
3-[(2S)-2-Pyrrolidinyl]pyridine
| |
| Other names
Demethylnicotine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.165.066 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H12N2 | |
| Molar mass | 148.209 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nornicotine is an alkaloid found in various plants including Nicotiana, the tobacco plant. It is chemically similar to nicotine, but does not contain a methyl group.
It is a precursor to the carcinogen N-nitrosonornicotine that is produced during the curing and processing of tobacco.[1]
Synthesis
For the synthesis of nornicotine several paths can be taken. This includes the demethylation of nicotine. The removal of the methyl group can be accomplished for example by reaction with silver oxide.[2]


The reduction of 3-myosmine for example with molecular hydrogen on palladium-carbon catalyst[3] or with sodium borohydride[4] provides (±) -Nornicotin in moderate to good yields.

References
- ^ Siminszky, B. (2005). "Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase". Proceedings of the National Academy of Sciences. 102 (41): 14919–24. doi:10.1073/pnas.0506581102. PMC 1253577. PMID 16192354.
- ^ Spaeth, Chem. Ber. 1936, 69, 250–251.
- ^ Haines, J. Am. Chem. Soc. 1945, 67, 1258–1260.
- ^ T. J. Dickerson, K. D. Janda; J. Am. Chem. Soc. 2002, 124, 13, 3220–3221; PMID 11916401.

![{\displaystyle \mathrm {\xrightarrow[{H2O}]{Ag2O}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/b5c635f4bc8d7e7749e6712a46c069a72be9beaf)
![{\displaystyle \mathrm {\xrightarrow[{H2}]{Pd/C}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/41e928ce8ffd207a9b8a18f2d82aa25e79a8e2b7)