Norrish reaction
The Norrish reaction in organic chemistry describes the photochemical reactions taking place with ketones and aldehydes. This type of reaction is subdivided in Norrish type I reactions and Norrish type II reactions.[1] The reaction is named after Ronald George Wreyford Norrish.
Type I
The Norrish type I reaction is the photochemical cleavage or homolysis of aldehydes and ketones into two free radical intermediates. The carbonyl group accepts a photon and is excited to a photochemical singlet state. Through intersystem crossing the triplet state can be obtained. On cleavage of the α-carbon bond from either state, two radical fragments are obtained.[2] The size and nature of these fragments depends upon the stability of the generated radicals; for instance, the cleavage of 2-butanone largely yields ethyl radicals in favor of less stable methyl radicals.[3]
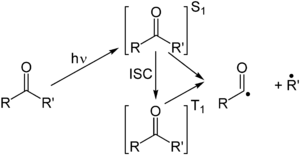
Several secondary reaction modes are open to these fragments depending on the exact molecular structure.
- The fragments can simply recombine to the original carbonyl compound, with racemisation at the α-carbon.
- Two organic residues can recombine with formation of a new carbon-carbon bond, with the loss of carbon monoxide.[2] The rate and yield of this product depends upon the bond-dissociation energy of the ketone's α substituents. Typically the more α substituted a ketone is, the more likely the reaction will yield products in this way.[4][5]
- The abstraction of an α-proton from the carbonyl fragment may form a ketene and an alkane.
- The abstraction of a β-proton from the alkyl fragment may form an aldehyde and an alkene.

The synthetic utility of this reaction type is limited, for instance it often is a side reaction in the Paternò–Büchi reaction. One organic synthesis based on this reaction is that of bicyclohexylidene.[6]
Type II
A Norrish type II reaction is the photochemical intramolecular abstraction of a γ-hydrogen (a hydrogen atom three carbon positions removed from the carbonyl group) by the excited carbonyl compound to produce a 1,4-biradical as a primary photoproduct.[7] Norrish first reported the reaction in 1937.[8]

Secondary reactions that occur are intramolecular recombination of the two radicals to a substituted cyclobutane in the Norrish–Yang reaction,[9] or fragmentation to an enol and an alkene.
Scope
The Norrish reaction has been studied in relation to environmental chemistry with respect to the photolysis of the aldehyde heptanal, a prominent compound in Earth's atmosphere.[10] Photolysis of heptanal in conditions resembling atmospheric conditions results in the formation of 1-pentene and acetaldehyde in 62% chemical yield together with cyclic alcohols (cyclobutanols and cyclopentanols) both from a Norrish type II channel and around 10% yield of hexanal from a Norrish type I channel (the initially formed n-hexyl radical attacked by oxygen).
In one study [11] the photolysis of an Acyloin derivative in water in presence of hydrogen tetrachloroaurate (HAuCl4) generated nanogold particles with 10 nanometer diameter. The species believed to responsible for reducing Au3+ to Au0 [12] is the Norrish generated ketyl radical.

No fewer than three Norrish-type reactions feature in the classic 1982 total synthesis of dodecahedrane.
An example of a synthetically useful Norrish type II reaction can be found early in the total synthesis of the biologically active cardenolide ouabagenin by Baran and coworkers.[13] The optimized conditions minimize side reactions, such as the competing Norrish type I pathway, and furnish the desired intermediate in good yield on a multi-gram scale.

See also
References
- ^ Named Organic Reactions, 2nd Edition, Thomas Laue and Andreas Plagens, John Wiley & Sons: Chichester, England, New York, 2005. 320 pp. ISBN 0-470-01041-X
- ^ a b "IUPAC Gold Book - Norrish Type I photoreaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.N04219. Retrieved 31 March 2014.
- ^ Blacet, F. E.; N. Pitts Jr., James (1950). "Methyl Ethyl Ketone Photochemical Processes". Journal of the American Chemical Society. 72 (6): 2810–2811. doi:10.1021/ja01162a544.
- ^ Yang, Nien-Chu; D. Feit, Eugene; Hui, Man Him; Turro, Nicholas J.; Dalton, Christopher (1970). "Photochemistry of di-tert-butyl ketone and structural effects on the rate and efficiency of intersystem crossing of aliphatic ketones". Journal of the American Chemical Society. 92 (23): 6974–6976. doi:10.1021/ja00726a046.
- ^ Abuin, E.B.; Encina, M.V.; Lissi, E.A. (1972). "The photolysis of 3-pentanone". Journal of Photochemistry. 1 (5): 387–396. doi:10.1016/0047-2670(72)80036-4.
- ^ Bicyclohexylidene Nicholas J. Turro, Peter A. Leermakers, and George F. Vesley Organic Syntheses, Coll. Vol. 5, p.297 (1973); Vol. 47, p.34 (1967) Online article.
- ^ "IUPAC Gold Book - Norrish Type II photoreaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.N04218. Retrieved 31 March 2014.
- ^ Norrish, R. G. W.; Bamford, C. H. (31 July 1937). "Photo-decomposition of Aldehydes and Ketones". Nature. 140: 195–6. doi:10.1038/140195b0.
- ^ "IUPAC Gold Book - Norrish–Yang reaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.NT07427. Retrieved 31 March 2014.
- ^ Photolysis of Heptanal Suzanne E. Paulson, De-Ling Liu, Grazyna E. Orzechowska, Luis M. Campos, and K. N. Houk J. Org. Chem.; 2006; 71(17) pp 6403 - 6408; (Article) doi:10.1021/jo060596u
- ^ Facile Photochemical Synthesis of Unprotected Aqueous Gold Nanoparticles Katherine L. McGilvray, Matthew R. Decan, Dashan Wang, and Juan C. Scaiano J. Am. Chem. Soc.; 2006; 128(50) pp 15980 - 15981; (Communication) doi:10.1021/ja066522h
- ^ Technically Au3+ is reduced to Au2+ which then forms Au+ and Au3+ by disproportionation followed by final reduction of Au1+ to Auo
- ^ Renata, H.; Zhou, Q.; Baran, P. S. (3 January 2013). "Strategic Redox Relay Enables A Scalable Synthesis of Ouabagenin, A Bioactive Cardenolide". Science. 339 (6115): 59–63. doi:10.1126/science.1230631.
